Co-Evolution of Microbes and the Mammalian Gut: Difference between revisions
No edit summary |
|||
| Line 31: | Line 31: | ||
<br>Study of the relationship between the gut microbiota in The desert woodrat (<i>N. lepida</i>), and the animals tolerance to PSCs on the creosote bush in the mojave desert, is well doccumented<ref name=karasov1989>Karasov, William H. "Nutritional bottleneck in a herbivore, the desert wood rat (Neotoma lepida)." Physiological Zoology 62.6 (1989): 1351-1382.</ref><ref name=kohl2012>Kohl, Kevin D., and M. D. Dearing. "Experience matters: prior exposure to plant toxins enhances diversity of gut microbes in herbivores." Ecology letters 15.9 (2012): 1008-1015.</ref><ref name=kohl2014>Kohl, Kevin D., et al. "Gut microbes of mammalian herbivores facilitate intake of plant toxins." Ecology Letters 17.10 (2014): 1238-1246.</ref>. The creosote bush (<i>Larrea tridentata</i>) is abundant in the Mojave desert, in the southern United States, because of its resistance to herbivores, drought and ageing <ref name=Yong2016>Yong, Ed. I contain multitudes: The microbes within us and a grander view of life. Random House, 2016.</ref>. The plant is covered in waxy chemicals that prevent water loss and deter predators. The oldest ring of cloned creosote bushes has been alive for over 11,000 years <ref name=vasek1980>Vasek, Frank C. "Creosote bush: Long‐lived clones in the Mojave Desert." American Journal of Botany 67.2 (1980): 246-255.</ref>. | <br>Study of the relationship between the gut microbiota in The desert woodrat (<i>N. lepida</i>), and the animals tolerance to PSCs on the creosote bush in the mojave desert, is well doccumented<ref name=karasov1989>Karasov, William H. "Nutritional bottleneck in a herbivore, the desert wood rat (Neotoma lepida)." Physiological Zoology 62.6 (1989): 1351-1382.</ref><ref name=kohl2012>Kohl, Kevin D., and M. D. Dearing. "Experience matters: prior exposure to plant toxins enhances diversity of gut microbes in herbivores." Ecology letters 15.9 (2012): 1008-1015.</ref><ref name=kohl2014>Kohl, Kevin D., et al. "Gut microbes of mammalian herbivores facilitate intake of plant toxins." Ecology Letters 17.10 (2014): 1238-1246.</ref>. The creosote bush (<i>Larrea tridentata</i>) is abundant in the Mojave desert, in the southern United States, because of its resistance to herbivores, drought and ageing <ref name=Yong2016>Yong, Ed. I contain multitudes: The microbes within us and a grander view of life. Random House, 2016.</ref>. The plant is covered in waxy chemicals that prevent water loss and deter predators. The oldest ring of cloned creosote bushes has been alive for over 11,000 years <ref name=vasek1980>Vasek, Frank C. "Creosote bush: Long‐lived clones in the Mojave Desert." American Journal of Botany 67.2 (1980): 246-255.</ref>. | ||
<br> Whilst the native population of <i>N. lepida</i> living in the Mojave desert are able to survive on little more than just creosote leaves over the winter and spring months, if a lab rat consumes too much, it dies<ref name=Rios2008>Ríos, Juan Manuel, Antonio Marcelo Mangione, and Jose Carlos Gianello. "Effects of natural phenolic compounds from a desert dominant shrub Larrea divaricata Cav. on toxicity and survival in mice." (2008).</ref>. Kevin Kohl and his colleagues investigated the differences between woodrat populations in the Mojave desert, where the Creosote bush is present, and the Great Basin Desert, where it is not<ref name=kohl2014>Kohl, Kevin D., et al. "Gut microbes of mammalian herbivores facilitate intake of plant toxins." Ecology Letters 17.10 (2014): 1238-1246.</ref>. Whilst the experienced, creosote eating, Mojave desert population of woodrats tends to express more detoxification enzymes<ref name=magnanou2009>Magnanou, E., J. R. Malenke, and M. D. Dearing. "Expression of biotransformation genes in woodrat (Neotoma) herbivores on novel and ancestral diets: identification of candidate genes responsible for dietary shifts." Molecular Ecology 18.11 (2009): 2401-2414.</ref>, they also have distinct microbial communities in their gut when compared to the woodrats native to the great basin<ref name=kohl2012>Kohl, Kevin D., and M. D. Dearing. "Experience matters: prior exposure to plant toxins enhances diversity of gut microbes in herbivores." Ecology letters 15.9 (2012): 1008-1015.</ref>. When Kohl fed the Mojave desert woodrats antibiotics, they were unable to feed on creosote resin and lost >10% of their body mass within 2 weeks, indicating that microbes are largely responsible for the rats' adaptable diet. Conversely, woodrats from the Great Basin population were able to consume only creosote resin whilst maintaining their body weight after recieving a fecal transplant from the experienced rats. Although the specific species responsible for these changes is not known, the antibiotic reduced the abundancy of [https://microbewiki.kenyon.edu/index.php/Spirochaeta Spirochaete] and Tenericute bacteria<ref name=kohl2014>Kohl, Kevin D., et al. "Gut microbes of mammalian herbivores facilitate intake of plant toxins." Ecology Letters 17.10 (2014): 1238-1246.</ref>. | <br> Whilst the native population of <i>N. lepida</i> living in the Mojave desert are able to survive on little more than just creosote leaves over the winter and spring months, if a lab rat consumes too much, it dies<ref name=Rios2008>Ríos, Juan Manuel, Antonio Marcelo Mangione, and Jose Carlos Gianello. "Effects of natural phenolic compounds from a desert dominant shrub Larrea divaricata Cav. on toxicity and survival in mice." (2008).</ref>. Kevin Kohl and his colleagues investigated the differences between woodrat populations in the Mojave desert, where the Creosote bush is present, and the Great Basin Desert, where it is not<ref name=kohl2014>Kohl, Kevin D., et al. "Gut microbes of mammalian herbivores facilitate intake of plant toxins." Ecology Letters 17.10 (2014): 1238-1246.</ref>. Whilst the experienced, creosote eating, Mojave desert population of woodrats tends to express more detoxification enzymes<ref name=magnanou2009>Magnanou, E., J. R. Malenke, and M. D. Dearing. "Expression of biotransformation genes in woodrat (Neotoma) herbivores on novel and ancestral diets: identification of candidate genes responsible for dietary shifts." Molecular Ecology 18.11 (2009): 2401-2414.</ref>, they also have distinct microbial communities in their gut when compared to the woodrats native to the great basin<ref name=kohl2012>Kohl, Kevin D., and M. D. Dearing. "Experience matters: prior exposure to plant toxins enhances diversity of gut microbes in herbivores." Ecology letters 15.9 (2012): 1008-1015.</ref>. When Kohl fed the Mojave desert woodrats antibiotics, they were unable to feed on creosote resin and lost >10% of their body mass within 2 weeks, indicating that microbes are largely responsible for the rats' adaptable diet. Conversely, woodrats from the Great Basin population were able to consume only creosote resin whilst maintaining their body weight after recieving a fecal transplant from the experienced rats. Although the specific species responsible for these changes is not known, the antibiotic reduced the abundancy of [https://microbewiki.kenyon.edu/index.php/Spirochaeta Spirochaete] and Tenericute bacteria<ref name=kohl2014>Kohl, Kevin D., et al. "Gut microbes of mammalian herbivores facilitate intake of plant toxins." Ecology Letters 17.10 (2014): 1238-1246.</ref>. | ||
<br><br>Behavioural adaptations of herbivores may help them to alter their gut microbiomes in order to cope with plant toxins. Many mammals, including desert woodrats, are known to engage in geophagia (soil consumption) in order to absorb toxins<ref name=krishnamani2000>Krishnamani, Ramanathan, and William C. Mahaney. "Geophagy among primates: adaptive significance and ecological consequences." Animal behaviour 59.5 (2000): 899-915.</ref>. This is a possible source of detoxifying bacteria, as soil contains many microbes that are able to degrade phenolic compounds<ref name=blum1988>Blum, Udo, and Steven R. Shafer. "Microbial populations and phenolic acids in soil." Soil Biology and Biochemistry 20.6 (1988): 793-800.</ref>. | |||
==Conclusion== | ==Conclusion== | ||
Revision as of 14:19, 22 April 2020
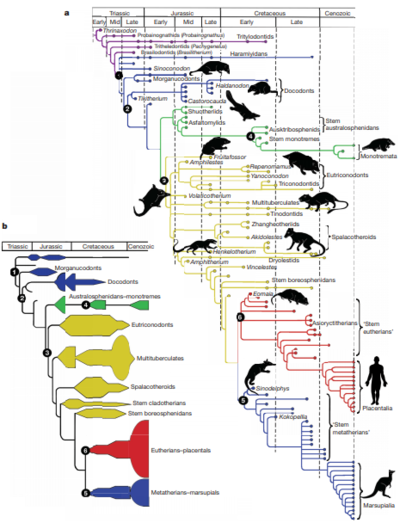
Introduction
By Joanna van Dyk
Microbes likely had commensal relationships with the ancestors of mammals, long before they evolved to give birth to live young or obtained many of the traits that characterize the class of vertebrates today. [2] The digestive tract of a mammal provides a perfect environment for microbial colonization, it is moist, warm, and has a continuous supply of food. The microbes, in turn, are able to produce toxin-digesting enzymes that allow their hosts to consume a wide variety of nutrients[3]. In fact, vertebrate guts have provided such a unique and hospitable habitat for microbial colonization, that microbial gut populations are more distinct from "external" habitats (such as lakes and soils) than these habitats are from each other[2]. Today, the microbiota of mammalian guts show similarities between species from similar ancestry, but also between those that have similar diets[2].
The earliest mammals were carnivorous, and without microbes, they would have not been able take advantage of the diverse array of nutrients offered by the plant kingdom. This allowed them to fill many of the ecological niches left when the dinosaurs became extinct 65 million years ago.[4] Mammalian gut microbiomes have evolved to aid their hosts in the digestion of plant toxins that would otherwise completely disable them, such as Nordihydroguaiaretic acid. This chemical would cause desert woodrats (N. lepida) to develop kidney cysts and liver damage if its gut bacteria did not digest it for them. [5] Unlike other vertebrates, mammals are in the unique position of being able to inherit their microbiomes from their mothers through the vaginal canal and through breast milk. This may enable the preservation of microbial lineages over time[6]
In this page we adress the questions: How specialized are the microbe lineages associated with mammalian guts? And, when a mammal adopts a diffferent diet, how much does its gut microbiome resemble the microbiomes of its close relatives?
Early Evolution
Colonization of the Gut
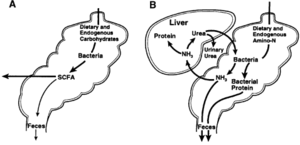
Hindgut and Foregut Fermentation
Ruminants utilize bacteria such as Ruminococcus to digest their food.
Detoxification of Plant Secondary Compounds
Unlike animals, plants cannot run away from other organisms trying to consume them. Whilst moving prey have evolved fast running legs and meticulous camoflage to evade predators, plants must rely on chemical compounds to avoid being eaten. Plant toxins are diverse, and can do anything to consumers from causing mild digestive discomfort, to triggering abortions and death [4]. These compounds are known as plant secondary metabolites (PSMs), and they have a significant impact on the nutritional ecology of mammals that eat them[3].Whilst herbivores can develop enzymes to digest toxins themselves, mammalian genomes are far slower to evolve than those of tiny, fast-replicating bacteria. Microbes are able to digest almost any compound on the planet[9], and since they evolve so quickly and can horizontally transfer genes between species, they are able to occupy any ecological niche within the mammalian gut. This may be one of the reasons that herbivorous mammals have been so successful in many earth ecosystems[4]. However, studies that specifically address the microbiome as a driver of diet diversification in herbivores have been inconclusive[10].
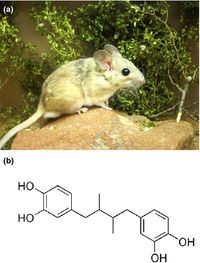
Detoxification of Creosote resin in Desert Woodrats
Study of the relationship between the gut microbiota in The desert woodrat (N. lepida), and the animals tolerance to PSCs on the creosote bush in the mojave desert, is well doccumented[11][10][5]. The creosote bush (Larrea tridentata) is abundant in the Mojave desert, in the southern United States, because of its resistance to herbivores, drought and ageing [4]. The plant is covered in waxy chemicals that prevent water loss and deter predators. The oldest ring of cloned creosote bushes has been alive for over 11,000 years [12].
Whilst the native population of N. lepida living in the Mojave desert are able to survive on little more than just creosote leaves over the winter and spring months, if a lab rat consumes too much, it dies[13]. Kevin Kohl and his colleagues investigated the differences between woodrat populations in the Mojave desert, where the Creosote bush is present, and the Great Basin Desert, where it is not[5]. Whilst the experienced, creosote eating, Mojave desert population of woodrats tends to express more detoxification enzymes[14], they also have distinct microbial communities in their gut when compared to the woodrats native to the great basin[10]. When Kohl fed the Mojave desert woodrats antibiotics, they were unable to feed on creosote resin and lost >10% of their body mass within 2 weeks, indicating that microbes are largely responsible for the rats' adaptable diet. Conversely, woodrats from the Great Basin population were able to consume only creosote resin whilst maintaining their body weight after recieving a fecal transplant from the experienced rats. Although the specific species responsible for these changes is not known, the antibiotic reduced the abundancy of Spirochaete and Tenericute bacteria[5].
Behavioural adaptations of herbivores may help them to alter their gut microbiomes in order to cope with plant toxins. Many mammals, including desert woodrats, are known to engage in geophagia (soil consumption) in order to absorb toxins[15]. This is a possible source of detoxifying bacteria, as soil contains many microbes that are able to degrade phenolic compounds[16].
Conclusion
References
- ↑ Luo, Zhe-Xi. "Transformation and diversification in early mammal evolution." Nature 450.7172 (2007): 1011-1019.
- ↑ 2.0 2.1 2.2 Ley, Ruth E et al. “Worlds within worlds: evolution of the vertebrate gut microbiota.” Nature reviews. Microbiology vol. 6,10 (2008): 776-88. doi:10.1038/nrmicro1978
- ↑ 3.0 3.1 Dearing, M. Denise, William J. Foley, and Stuart McLean. "The influence of plant secondary metabolites on the nutritional ecology of herbivorous terrestrial vertebrates." Annual review of ecology, evolution, and systematics 36 (2005)
- ↑ 4.0 4.1 4.2 4.3 Yong, Ed. I contain multitudes: The microbes within us and a grander view of life. Random House, 2016.
- ↑ 5.0 5.1 5.2 5.3 5.4 Kohl, Kevin D., et al. "Gut microbes of mammalian herbivores facilitate intake of plant toxins." Ecology Letters 17.10 (2014): 1238-1246.
- ↑ Kelley, Scott T., and Susanne Dobler. "Comparative analysis of microbial diversity in Longitarsus flea beetles (Coleoptera: Chrysomelidae)." Genetica 139.5 (2011): 541-550.
- ↑ Stevens, C. Edward, and Ian D. Hume. "Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients." Physiological reviews 78.2 (1998): 393-427.
- ↑ Grajal, A., and S. D. Strahl. "A bird with the guts to eat leaves." Natural History 8 (1991): 48.
- ↑ Leahy, Joseph G., and Rita R. Colwell. "Microbial degradation of hydrocarbons in the environment." Microbiology and Molecular Biology Reviews 54.3 (1990): 305-315
- ↑ 10.0 10.1 10.2 Kohl, Kevin D., and M. D. Dearing. "Experience matters: prior exposure to plant toxins enhances diversity of gut microbes in herbivores." Ecology letters 15.9 (2012): 1008-1015.
- ↑ Karasov, William H. "Nutritional bottleneck in a herbivore, the desert wood rat (Neotoma lepida)." Physiological Zoology 62.6 (1989): 1351-1382.
- ↑ Vasek, Frank C. "Creosote bush: Long‐lived clones in the Mojave Desert." American Journal of Botany 67.2 (1980): 246-255.
- ↑ Ríos, Juan Manuel, Antonio Marcelo Mangione, and Jose Carlos Gianello. "Effects of natural phenolic compounds from a desert dominant shrub Larrea divaricata Cav. on toxicity and survival in mice." (2008).
- ↑ Magnanou, E., J. R. Malenke, and M. D. Dearing. "Expression of biotransformation genes in woodrat (Neotoma) herbivores on novel and ancestral diets: identification of candidate genes responsible for dietary shifts." Molecular Ecology 18.11 (2009): 2401-2414.
- ↑ Krishnamani, Ramanathan, and William C. Mahaney. "Geophagy among primates: adaptive significance and ecological consequences." Animal behaviour 59.5 (2000): 899-915.
- ↑ Blum, Udo, and Steven R. Shafer. "Microbial populations and phenolic acids in soil." Soil Biology and Biochemistry 20.6 (1988): 793-800.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2018, Kenyon College.
