Eyes: Difference between revisions
(New page: {{Biorealm Niche}} This template is a general guideline of how to design your site. You are not restricted to this format, so feel free to make changes to the headings and subheadings an...) |
No edit summary |
||
| (253 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Uncurated}} | ||
[[Image:eye.jpg|thumb|The Human Eye|300px|right|The Human Eye]] | |||
[[image:NEA09.gif|thumb|350px|Anatomy of the Eye [http://www.nei.nih.gov/health/eyediagram/images/NEA09.gif].]] | |||
==Introduction== | |||
The eye, it has been said that the eyes are our window to the world. Of the five senses at our disposal, nothing more than the eyes have greatly infused us with an overwhelming abundance of the stimuli collected from the greater world. The eyes personify as the prevailing instrument that shapes our very individuality. Our perceptions, our interactions, our very definitions of who we are, stem from the visual cues received, processed and reflected back. Hence the eyes have been referred to as the window into our soul. | |||
The evolution of the eyes dates back to the pre-Cambrian explosion when the first proto-eyes emerged. These simplest eyes used to detect light and darkness have evolved into wide ranging complexities and have emerged into myriad forms found today. From the compound eyes found in arthropods with its many facets permitting a wide ranging 360 degree field of vision, to the common single lens eyes that telescope and modulate to generate images, to eyes capable of registering light wavelength in the ultraviolet spectrum, the eyes today are all about the ever increasing information gathering, to provide a better edge and thus enhance survivability. [http://ebiomedia.com/gall/eyes/octopus-insect.html (36)] | |||
Our focus today looks not at the anatomy of the eye, but rather as an organ, the eye provide a niche for other organisms to explore, exploit and establish residence within its border. Specifically, we will deal primarily with the human eye and the conditions it provides as the a suitable host. We will also examine some of the defense mechanisms employed by the human body to maintain law and order and thus prevent of the over infestation by undesirable colonization of microorganisms. | |||
== | ==The Eye Niche== | ||
===Physical | ===Physical Location of the Eyes=== | ||
[[image:Optic.jpg|thumb|350px|The Optic foraman [http://en.wikipedia.org/wiki/Image:Orbita_mensch.jpg].]] | |||
The human eye is located in the anterior portion of the cranium. The eyes are housed within the 7 bones that form the orbit of the eye; the frontal bone, the maxilla, the zygomatic bone, the lacrimal bone, the nasal bone (not part of orbit), the sphenoid bone, the ethmoid bone and the palatine bone. [http://education.yahoo.com/reference/gray/subjects/subject/35#p147 (37)] | |||
=== | ===Physical features of the Human Eye=== | ||
The eye is comprised of three distinct layers. The tunica fibrosa oculi is the outer layer fabricated from the collagen. Its functions include protection of the eye and serve to give the overall round shape of the eyeball. The region also consist of the sclera and cornea. The tunica vasculosa oculi represents the middle layer of the eye. Its vascular region is responsible for the transport of nutrients, wastes, immunoglobulins, and the more important gas exchange of oxygen and carbon dioxide. Amongst the noticeable features of this region is the iris, ciliary body, and the choroid (which are blood vessels responsible for the mentioned functions). The tunica nervosa oculi is the innermost region of the three layers, it is sensory portion of the eye containing rods and cones on the retina. The impulses generated from reactions with light waves are sent to the brain via the optic nerve. [http://academia.hixie.ch/bath/eye/home.html (38)] | |||
In addition the eye is subdivided into the anterior and posterior chambers. the anterior portion includes the structures in front of the vitreous humour, these are the cornea, the iris, the ciliary body, and the lens. The anterior chamber is bathed in a replenishing aqueous humor. The larger portion is the posterior chamber. These involve the anterior hyaloid membrane and all of the optical structures behind it,the vitreous humor, retina, choroid, and optic nerve. [http://www.clinica-cotero.es/i/2-1-1.htm (39)] | |||
===Conditions of the Eye=== | |||
Normal pH range in the human eye ranges from 7.0 to 7.4 (very neutral). | |||
Normal eyes closed temperature = 34.77′0.37 °C | |||
Normal eyes open temperature = 35.02′0.39 °C | |||
The avascular nature of the eye heavily depends on the aqueous humor and the vitreous humor to transport nutrients, wastes, and gas exchanges. Containing the right pH, temperature and proper nutrients, the eye is a great and prized niche for microorganisms. | |||
The aqueous humor is a replenishing solution filled the area between the lens and the cornea. The aqueous humor establishes intraocular pressure of the anterior chamber, resulting in the bulgeoning shape of the eye. In addition, the solution carries nutrition in forms of amino acids and glucose for the avascular ocular tissues namely the posterior cornea, trabecular meshwork, lens, and anterior vitreous. Second, the aqueous humor removes waste products from metabolism of the above avascular ocular tissues. Immunoglobulins provide an immune response to defend against pathogens. | |||
The vitreous humor is a gelatinous mass that occupies the space between the lens of the eye and the retina. The vitreous humor generates the intraocular pressure ranging from 11-21 mm of Hg. The gel in the vitreous chamber is stagnant. Hence there is a high concentration of phagocytes which remove cellular debris impeding the visual field. The vitreous humor contains no blood vessels with 99% of its volume made up of a solution of mostly water and low concentrations of; salts, sugars, vitrosin, as well as a network of collagen. [http://svconline.com/mag/avinstall_eye_ear_brain/index.html (40)] | |||
===Conditions under which the environment changes=== | ===Conditions under which the environment changes=== | ||
The eye is a very controlled environment. Thus any changes in chemical concentrations, infiltration of the niche by other microorganisms, or trauma to the eye may affect the natural community of the eye niche. Due to the mechanisms that maintain sterile conditions of the eye, disturbances from microorganism, trauma or chemicals may set off an inflammatory immune responses characterized by mild symptoms such as irritation to more serious problems such as diminishing eye sight. | |||
===Defense Mechanisms in the Eye=== | |||
Barely any microbes live in the eye. This stems from the fact that each section within the eye has mechanisms that defend against infection and microbial colonization. | |||
'''Blink Reflex''' | |||
The blink reflex is a mechanical defense against particles in the air or trauma. Eyelashes and the sensitive cornea both participate in this reflex. Tears, debris, allergens, microbes, etc. are moved over to the lacrimal excretory system with the motion from the eyelid. | |||
'''Barriers''' | |||
The orbital septum, cornea and conjunctiva all provide a protective barrier against pathogens. The orbit and the eyelid are separated into preseptal and postseptal spaces by the orbital septum which creates a physical barrier against infections. The various layers of the cornea limit permeability of items into the eye. Also, native flora of the lids and mucosal surface limit possible pathogenic colonization. [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88956 (10)] | |||
'''Tears''' | |||
Tears provide a mechanical defense via flushing of foreign particles from the surface of the eye and transporting antimicrobial agents to the surface as defensive measures. [http://www.nature.com/eye/journal/v17/n8/full/6700617a.html (11)] | |||
'''Immune Response''' | |||
The cornea, due to lack of a vascular system, contains limited immune defenses. Immune defenses are provided by Langerhans cells and immunoglobulins. Langerhans cells modify B and T cell in the cornea while glycocalyx [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TCC-44CHKR8-3&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=4429&md5=ecd1d84e68b637836d809a5a8c952bb9 (13)], a transmembrane mucin made up of a mucin-like glycoprotein adds protection by preventing adhesion of foreign molecules to the surface of the eye. | |||
Langerhans cells detect immunoglobulins, antigen, and complement. They work like macrophages to destroy foreign invaders. The helper B and T cells that are modified by Langerhans cells work in conjunction with lymphocytes to elicit an immune response. [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88956 (10)] | |||
'''Leukocyte Defense''' | |||
Leukocytes consume and destroy microorganisms via and oxygen-dependent pathway and an oxygen-independent pathway. The oxygen-dependent pathway uses oxygen radicals while the oxygen-independent pathway utilizes defensins which are peptides that have a broad range of antibacterial, antifungal, and some antiviral activities. [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88956 (10)] [http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=2997278 (12)] | |||
==Eyelids== | |||
[[image:stye.jpg|thumb|350px|Stye on Eye [http://www.uic.edu/com/eye/LearningAboutVision/EyeFacts/Images/stye.jpg].]] | |||
The eyelids are the protective folds to the eyes, which are continuous with the adjacent skin. They consist essentially of an outer sheet of muscle, orbicularis, and an inner fibrous, tarsal plate, covered by skin on the outer side and conjunctiva on the inside. | |||
Although most bacteria found around the eyes usually live harmlessly in the eye niche, they can still cause infections and irritations to the eye. Some of the most common bacterial infections in the eyelid are caused by bacteria from the Staphylococcus family, such as ''[[Staphylococcus aureus]]'' and ''[[Staphylococcus epidermidis]]''. They are harmless under normal conditiona, but they can cause diseases such as blepharitis or hordeola (stye) by entering wounded skin on the eyelids. [http://www.sfam.org.uk/pdf/features/bugeyed.pdf (31)] | |||
Many eye allergies are followed by bacterial infections. Demodex folliculorum is a tiny parasitic mite that lives in or near the hair follicle of mammals and it can cause allergic reaction which leads to the scaling of epidermis, destructions of the eyelash follicles and formation of ulcers. In many cases hordeola follows after blepharitis. | |||
Most people get staph infections by physical contact with contaminated objects. Example such as Hordeolum (stye), is a pustule on the eyelid margins commencing within the follicle of an eyelash or in one of the associated glands caused by presence of ''[[Staphylococcus aureus]]''. [http://www.emedicine.com/oph/TOPIC606.HTM (34)] | |||
Eye cosmetic products are one of many things that can be contaminated with bacteria. They possess nutrients such as lipids, polysaccharides, amino acids, and glycosides...etc, that will facilitate growth of microorganism. With the wrong application method, sometimes the user can break the skin, which leads to staph bacteria entering the wound and causes inflammations and other eye diseases. (30) | |||
==Tears== | |||
Tears are one of the most important defense systems that our eyes have. Tears are secretion from tear glands that lubricate and protect eyes from infection. The tear film allows gas exchange between the air and the epithelium and also keeps the cornea wet. The substance of the tear film is retinol to provide the transparent nature of the epithelium. [http://www.nature.com/eye/journal/v17/n8/full/6700566a.html (16)] | |||
Tear fluid is composed of water, electrolytes (sodium and potassium), proteins (immunoglobulin and lysozyme), lipid, mucins, and others such as lactoferrin, lipocalin, and lacritin. | |||
These components of tears help form a defense system that guards the eyes from infection. For example, IgA is the main immunoglobulin of tears and is synthesized by subconjunctival plasma cells. Because of the dimer structure of this protein, it is very effective in aggregating targets to hampers their penetration of the body surface [http://www.lib.mcg.edu/edu/esimmuno/ch3/biologic.htm (5)]. Besides immunoglobulin, another protein called lysozyme has similar functions. “Lysozyme is an enzyme that is naturally abundant in tears and plays an important role in maintaining healthy eyes” [http://www.lion.co.jp/en/press/html/2007017f.htm (6)]. Lysozymes are known to have anti-inflammatory and bacteriolytic functions because they can dissolve the outer coating of certain bacteria; and thus, killing the bacteria so that it cannot infect the eye. | |||
In addition, lactoferrin is an iron binding protein secreted by the epithelial acinar cells of the lacrimal glands. Lactoferrin is one of the key molecules that regulate the inflammatory response and the protection against infections. The sequestration of iron by lactoferrin can firmly control bacterial flora and inhibits the iron catalyzed production of hydroxyl radical. Generally, hydroxyl radical may lead to major tissue damage by producing peroxidation of cell membrane lipids, oxidative damage to proteins, and generation of other free radicals. Hence, lactoferrin’s ability to deplete free iron can inhibits the pro-inflammatory effects of hydroxyl radical; and therefore, protects the mucosal surfaces from oxidative damage. Moreover, lactoferrin inhibits the ability of bacteria and viruses to attach to cell membrane of the host cell [http://www.touchscientific.com/lfnfact2.htm (7)]. | |||
Furthermore, lipocalin is another component of tears that form the eye’s defense system. Lipocalin is the major lipid binding protein in tears. Lipocalin can bind to wide variety of lipids such as fatty acids, cholesterol, phospholipids and glycolipids. By interacting with lipids, tear lipocalin can contribute to the surface pressure in the tear film. Moreover, lipocalin also help to promote optical clarity and may transport lipids from the lacrimal gland to the tear film. It plays a significant role in the protection of the ocular surface from desiccation because the lipids that are bounded to the tear lipocalin can serve to provide a reservoir of lipid molecules in equilibrium with the surface and could reduce evaporation of water. Moreover, tear lipocalin can gather lipid from the cornea to prevent dry spots from forming on the cornea [http://www.missionforvisionusa.org/content/2005/12/dry-eye-disease.html (8)]. | |||
Likewise, lacritin also help promote ocular surface wetting. Lacritin flows onto the ocular surface from the lacrimal and meibomian glands. It is capable of protecting epithelia of the lacrimal-corneal axis against inflammation that associated with cell death. | |||
===Tear production system=== | |||
The protection of tears has three separate layers: the lipid layer, the aqueous layer, and the mucous layer. The outermost lipid layer is a thin layer (0.1-0.2µm that is composed of hydrophobic surface. It contains oils (lipids) produced by the meibomian glands. This oil (lipids) function to retain moisture and prevent tear fluid loss due to evaporation. The temperature in meilbomain gland lipids has the low melting point range 19-32°C depending on the degree of fatty acids unsaturation. The meibomain gland lipids allow lipid get through the meibomian gland ducts and excretion to the tear film. | |||
The aqueous layer is a tear film including water and proteins produced by the lacrimal gland. The middle layer of the lacrimal gland is the thickest (7-8µm) and produces 0.9% saline tears. It functions delivery the main nutrients such as oxygen to the cornea and carrying waste products away from the cornea | |||
The innermost mucous layer (30µm) contains mucin produced by goblet cells. The mucin provides a protective barrier for the surface and serves as a means for the aqueous layer to adhere to the surface of the eye. The mucous’s thin layer of hydrophilic surface can make even on the corneal surface to spread water. | |||
[http://physiologyonline.physiology.org/cgi/content/full/13/2/97 (17)] [http://www.tedmontgomery.com/the_eye/cornea.html (18)][http://www.lea-test.fi/en/eyes/lidsncha.html (19)] and [http://bjo.bmj.com/cgi/content/abstract/68/8/524 (20)] | |||
Conditions which affect the lipid layer or the mucin layer can cause a dry eye. | |||
[[image:lm.gif|none|200px|Anatomy of the Eye [http://www.matheson-optometrists.com/Products/Almera-Bausch-and-Lomb-Soothe.htm].]] People with dry eyes lack a complete lipid layer which result in an inadequate amount of tear fluid. Without this tear fluid, the person may experience a burning, gritty sensation and/or blurred vision. | |||
[[image:lr.gif|none|200px|Anatomy of the Eye [http://www.matheson-optometrists.com/Products/Almera-Bausch-and-Lomb-Soothe.htm].]] When lipids are restored, fluid is then replenished and moisture is restored in the eyes. [http://www.matheson-optometrists.com/Products/Almera-Bausch-and-Lomb-Soothe.htm (9)] | |||
Many species of bacteria are found in the eyelid and conjunctiva as symbiotic relationships. These are Staphylococcus aureus (S. aureus), Staphylococcus epidermidis, Streptococcus, Corynebacterium, Propionibacterium, and Propionibacterium acnes. These bacteria produce lipase that harm to the tear film lipid layer especially, S. epidermidis and S. aureus produce triglyceride lipase and cholesterol and wax esterase. Accumulated cholesterol is used for the growth of certain ocular bacteria. Increased bacteria are regulated by anti-microbial peptides from the lacrimal gland and conjunctiva. [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFD-49H6Y6X-1&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=10&md5=cae06adc263756d8bb890e5f40005b89#toc1 (21)] | |||
==Common bacteria found in Adjacent Communities== | |||
Ear: Staphylococcus (coagulase –neg.), Diptheroids, Pseudomonas spp., Enterobacteriaceae spp.. | |||
Nose: Staphylococcus (coagulase –neg.), S. aureus, Haemophilus spp., Viridians streptococci, S. pneumoniae | |||
Oropharyn: Staphylococcus (coagulase –neg.), S. aureus, Haemophilus spp., Viridians streptococci, S. pneumonia, Veilonella spp., Prevotella spp., Fusobacterium spp., Candida spp., Moraxella spp., Actinomyces, Eikenella corrodens. | |||
Skin: Staphylococcus (coagulase –neg.), S. aureus, Propionibacterium acnes, Haemophilus spp., Viridians streptococci, Mycobacterium spp., Bacillus spp., Candida spp. | |||
==Microscopic Organisms present in the Eye Niche== | |||
===Bacteria in the Eye Niche=== | |||
[[image:pseudomonas1.jpg|thumb|350px|Pseudomonas Aeruginosa [http://www.health.qld.gov.au/EndoscopeReprocessing/images/1314_pseudom.jpg].]] | |||
Many normal bacteria are present in the human eye which includes ''[[Staphylococcus]]'', ''[[Staphylococcus aureus]]'', Haemophilus species, and Streptococcus species. However, many other outside bacteria compete for their stay in the eye niche that may lead to tragic diseases and unfavaorable results. | |||
====Bacterial Effects on the Eye Environment via Contact Lens==== | |||
The most common bacteria that can cause corneal ulcer, which could lead to the development of eye blindness, are staphylococcal species, ''[[Pseudomonas aeruginosa]]'', ''[[Streptococcus pneumoniae]]'', and Moraxella species. Of these, ''[[Pseudomonas aeruginosa]]'' is found to be the primary bacterium when wearing contact lens, typically soft contacts, that can cause corneal ulcer. ''[[Pseudomonas aeruginosa]]'' is a gram-negative, aerobic rod-shaped bacterium which optimally grows at 42 degrees celcius. Although classified as aerobic, it can adapt and live in anaerobic environments. Being an opportunist, this bacterium greatly affects the eye when the defensive mechanisms of the eye are weakened. Otherwise, it does not ordinarily cause disease. It is, however, a very durable bacterium, some of which includes minimal food requirement, a protective outer coating, and resistance to many antibiotics [http://www.healthatoz.com/healthatoz/Atoz/common/standard/transform.jsp?requestURI=/healthatoz/Atoz/ency/pseudomonas_infections.jsp (22)]. | |||
Dr. Gifford Jones of canadafreepress.com had reported that people who wore contact lens are at risk of developing blinding eye infections during the first six months of use. He proposed that it depended on what the contact lenses were made of. It was said that ''[[Pseudomonas aeruginosa]]'' infection for soft contact lenses is one in 2,500 and one in 500 if it was worn overnight. For hard contact lenses, infection is one in 10,000. Biodeposits build up on the contact lenses which can cause irritation and corneal ulcers. When contact lenses are worn, it covers up the cornea which restricts oxygen flow to underlying tissues. In an anaerobic environment, ''[[Pseudomonas aeruginosa]]'' is then able to reside properly. Dr. Dwight Cavanagh, a professor of Opthlalmology at the University of Texas Southern Medical School, stated that new cells on the cornea cannot be produced when the contact lenses cover it, which may decrease the thickness of the cornea by 10 percent [http://www.canadafreepress.com/medical/ear-nose-throat030203.htm (25)]. | |||
A study from the United Kingdom reported that people who wear soft contact lenses result in 8 times more of a chance for bacteria to invade in the cornea when they sleep than the people who only wear them while they are awake [http://www.emedicine.com/emerg/topic115.htm (24)]. | |||
In 1993, The Department of Ophthalmology at University of Patras medical School in Greece, performed an experiment to determine the clinical microbiological characteristics of corneal ulcers in contact lens wearers. Results showed that, of the ulcerative keratitis patients, 26.74% of them wore contacts. In addition, ''[[Pseudomonas aeruginosa]]'' was the most frequent isolated bacterium. This experiment concluded that the use of contact lens has a high risk factor for ''[[ulcerative keratitis]]'' [http://www.healthatoz.com/healthatoz/Atoz/common/standard/transform.jsp?requestURI=/healthatoz/Atoz/ency/pseudomonas_infections.jsp (26)]. | |||
In addition, a study in Sydney of 2003, Australia found that ''[[Pseudomonas aeruginosa]]'' produce quorum-sensing signal moleucules which contributed to the induction of the inflammatory response in ''[[Pseudomonas keratitis]]'' [http://www.ncbi.nlm.nih.gov/pubmed/18463485?ordinalpos=6&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum (27)]. | |||
====Resistance to ''[[Pseudomonas aeruginosa]]''==== | |||
''[[Pseudomonas aeruginosa]]'' in the eye is targeted by the use of an antibiotic called ''[[ciprofloxacin]]''. Current research shows that ''[[ciprofloxacin]]'' is very effective in treating ''[[ulcerative keratitis]]'', although there have been rare results of the bacterium being resistant to the antibiotic, possibly due to the bacterium’s adaptation [http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1920786 (28)]. ''[[Pseudomonas aeruginosa]]'' has increased its resistance to many antibiotics up to today. Patients who are treats ''[[Pseudomonas aeruginosa]]'' often have to take multiple antibiotics due to the bacterium’s high barrier of antibiotic resistance [http://www.healthatoz.com/healthatoz/Atoz/common/standard/transform.jsp?requestURI=/healthatoz/Atoz/ency/pseudomonas_infections.jsp (23)]. | |||
===Fungal Infestation of the Eye Niche=== | |||
[[image:jcm00170-0184-a.jpg|thumb|350px|Fusarium solani [http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=273608&blobname=jcm00170-0184-a.jpg].]] | |||
Common fungi found in the Eye: | |||
- ''[[Acremonium spp]]''. | |||
- ''[[Aspergillus flavus]]'' | |||
- ''[[Aspergillus fumigatus]]'' | |||
- ''[[Aspergillus niger]]'' | |||
- ''[[Bipolaris spp.]]'' | |||
- ''[[Candida albican]]'' | |||
- ''[[Curvularia spp]]'' | |||
- ''[[Exserohilum spp.]]'' | |||
- ''[[Fusarium oxysporum]]'' | |||
- ''[[Fusarium solani]]'' | |||
- ''[[Lasiodiplodia theobromae]]'' | |||
The warm and moist environment of the eye is subject to mycotic infections from fungal species such as Fusarium, Aspergillus, Acremonium, and the Candida species of yeasts. Due to the preferences for moisture-rich environments and warm weather, the prevalence of fungal infections of the eye is higher in tropical areas and in areas with large agrarian communities (3). Despite the relatively sterile environment of the eye, factors such as eye “trauma (generally with plant material), chronic ocular surface diseases, contact lens usage, corneal anesthetic abuse and immunodeficiencies” can result in infection of the eye by filamentous fungi or yeasts (2). ''[[Fusarium solani]]'', a species of fungi that is associated with contact lens wearers, normally inhabits warmer climates in soil and organic matter. Invasion of the inner layers of contact lenses and transfer to the surface of the eye, however, is possible due to the ability of ''[[Fusarium solani]]'' to reside in the moisture-rich inner matrix of soft contact lenses. | |||
=== | ====Fungal Effects on the Eye Environment==== | ||
Fungal infections, such as fungal keratitis, can occur on the anterior surface of the eye or the cornea, and may also infect the posterior of the eye as a result of mycosis. Aggregation of fungal microbes into hyphae enlarges the infected region and prevents host inflammatory cells from ingesting the fungi. As a result of the pattern of fungi to join together and form hyphae, fungi that inhabit the eye may extend throughout the entire depth of the cornea, and tend to grow parallel to the lamellae of the cornea (1) (2). | |||
=== | ====Fungal Metabolism that Affects the Eye Niche==== | ||
Fungi that inhabit the eye produce proteases that are specific for the surface on which they grow. As a result, protein catabolism by fungal proteases results in tissue damage to the eye, ulceration as well as an inflammatory response around the infected area (1). As a result of catabolic degradation of the eye environment by the fungi, an inflammatory response by the host is accompanied by necrosis of the surrounding eye tissues. | |||
=== | ====Fungal Infection of the Eye Niche via Contact Lenses==== | ||
Fungal infection of the eye may occur as a result of fungal growth on contact lenses. Due to tendency of fungi to inhabit moisture-rich locations, contact lens cases and the soft inner matrix of contact lenses are subject to fungal growth. Fungi have the ability to degrade hydrophilic polymers of contact lenses, and may be transferred from contaminated lenses to the surface of the eye, often causing the implantation of fungi into the anterior regions of the eye or the cornea and result in fungal keratitis (1). | |||
==Closing Up the Eyes== | |||
The eye is an avascular organ bathed in an isotonic solution, which is exposed to the atmosphere and always in contact with microorganisms. Due to the environment and the defense mechanisms of the eye, very few microbes exist in the eye niche. Although the eye possesses natural defense mechanisms such as physical reflexes and immune system response, fungal microbes and bacterial microbes are still capable of infecting the eye. Many infections of the eye can be painful and if left untreated they can lead to more serious damages, so make sure you wash your hands before touching your eyes! | |||
==Current Research== | ==Current Research== | ||
1. Iris Pigment Epithelium Expressing CD86 (B7-2) Directly Suppresses T Cell Activation In Vitro via Binding to Cytotoxic T Lymphocyte–associated Antigen 4 | |||
A current study at Schepens Eye Research Insitute at Harvard Medical School identified the thin layer of cells lining the iris of the eye, the iris pigment epithelium (IPE), as a tissue that contributes to ocular-immune privilege by secreting immunosuppressive factors and expressing surface molecules that trigger apoptosis by T cells. Scientists exposed PE cells from various regions of the eye to purified T Cells and found that only the IPE cells from the iris responded to CD86 by stopping T cell activity. Normally, T cell activity in the body promotes inflammation. By suppressing its activity, however, IPE cells are able to prevent inflammation in the eye. The results demonstrated that IPE cells contribute to immune privilege and prevent eye inflammation in the iris. The study identified that disruption of IPE cell activity in the eye, perhaps by infection or other factors, may lead to immunogenic inflammation, disruption of the visual axis and blindness (4). | |||
2. Viral Infection of the Lungs through the Eye | |||
Respiratory syncytial virus (RSV) is a virus that is greatly known to affect the respiratory system of newborns. Researchers discovered an association of this virus with allergic conjunctivitis, also known as Pink Eye, which allowed the virus to flow toward the respiratory system initially from the eyes. They experimented on a live mouse by applying RSV to its eyes. RSV not only replicated but also drifted to the lungs, which produced a respiratory disease. Treatment of RSV with anticytokine was successful. They concluded that respiratory infections could be involved with the eye and that it can be treated. [http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1797451 (29)] | |||
3. Phospholipase A2 in Rabbit Tears: A Host Defense against Staphylococcus aureus | |||
Staphylococcus aureus causes keratitis in many human populations. | |||
Tear films have many nutrients that are beneficial for bacteria growth. Tear films also, however, have an effective defense system to reduce replication of bacteria and regulate bacterial growth. One of inhibitors for the host defense molecule is phosholipase A2 (PLA2). Moreau et al. studied the PLA2 effect on Staphylococcal activity. They found PLA2 killed Staphylococcus by hydrolyzing bacterial membranes to release fatty acids and destroyed the bacterial cell wall. [http://www.iovs.org/cgi/content/abstract/42/10/2347 (22)] | |||
4. Antimicrobial Contact Lenses | |||
Current research on antibacterial contact lenses to prevent microbial colonization into the eye niche, are going through various | |||
trials to measure the effectiveness of such lenses in comparison to regular lenses. The lenses were created to address the problem ofmicrobial contamination of lenses ranging from acute red eye to microbial keratitis. The lenses work by "jamming" the signaling systems that bacteria use to form biofilms. [http://www.sciencedaily.com/releases/2006/06/060629085000.htm (14)] The technology involves using a furanone technology to jam the signals and was discovered from a seaweed that generates the anti-biofilm compound. The drug-resistance problem of current antimicrobial agents should not affect these lenses since the technology is not designed to kill bacteria. [http://medgadget.com/archives/2006/06/biosignal_furan.html (15)] | |||
==References== | ==References== | ||
(1) Wilson LA, Ajello L. Agents of oculomycosis: fungal infections of the eye. In Collier L, Balows A, Sussman M, eds. Microbiology and microbial infections. 9th ed. Vol. 4, London, Arnold, 1998. | |||
(2) Tanure, M. A., E. J. Cohen, S. Sudesh, C. J. Rapuano, and P. R. Laibson. 2000. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 19:307-12. | |||
(3) L. Alcazar-Fuoli, E. Mellado, A. Alastruey-Izquierdo, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela Aspergillus Section Fumigati: Antifungal Susceptibility Patterns and Sequence-Based Identification Antimicrob. Agents Chemother., April 1, 2008; 52(4): 1244 - 1251. | |||
(4) Sugita, S., J. Streilein. “Iris Pigment Epithelium Expressing CD86 (B7-2) Directly Suppresses T Cell Activation In Vitro via Binding to Cytotoxic T Lymphocyte–associated Antigen 4.” J. Exp. Med., Jul 2003; 198: 161 - 171. | |||
(5) “Biological Functions of Immunoglobulins.” Structure, Function, & Genetics of Immunoglobulins. 20 Aug. 2008. <http://www.lib.mcg.edu/edu/esimmuno/ch3/biologic.htm> | |||
(6) “Lion to Release ‘Smile 40 Mediclear’.” Lion. 30 July 2007. 19 Aug. 2008. <http://www.lion.co.jp/en/press/html/2007017f.htm> | |||
(7) “Tear Lactoferrin.” 20 Aug. 2008. <http://www.touchscientific.com/lfnfact2.htm> | |||
(8) “Dry Eye Disease.” Mission for Vision – Complications of LASIK. 31 Dec. 2005. 19 Aug. 2008. <http://www.missionforvisionusa.org/content/2005/12/dry-eye-disease.html> | |||
(9) “Almera Soothe Eye Drops.” Matheson Optometrists. 25 Aug. 2008. | |||
<http://www.matheson-optometrists.com/Products/Almera-Bausch-and-Lomb-Soothe.htm> | |||
(10) Stephen A. Klotz, Christopher C. Penn, Gerald J. Negvesky, and Salim I. Butrus. Fungal and Parasitic Infections of the Eye. Clin Microbiol Rev. 2000 October; 13(4): 662–685. Accessed: 18 Aug 2008. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88956. | |||
(11) E K Akpek and J D Gottsch. Immune Defense at the Ocular Surface. Eye (2003). 28 Feb. 2003. 17, 949–956. Accessed: 18 Aug 2008. http://www.nature.com/eye/journal/v17/n8/full/6700617a.html | |||
(12) T Ganz, M E Selsted, D Szklarek, S S Harwig, K Daher, D F Bainton, and R I Lehrer. Defensins. Natural peptide antibiotics of human neutrophils. 1985 October; 76(4): 1427–1435. Accessed: 20 Aug 2008. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=2997278. | |||
(13) Maurizio Rolando MD and Manfred Zierhut MD. The Ocular Surface and Tear Film and Their Dysfunction in Dry Eye Disease. 6 Nov. 2001. Accessed: 27 Aug 2008. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TCC-44CHKR8-3&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=4429&md5=ecd1d84e68b637836d809a5a8c952bb9. | |||
(14) "First Human Trial of Antibacterial Contact Lenses." ScienceDaily. 29 June 2006. 27 Aug. 2008. <http://www.sciencedaily.com /releases/2006/06/060629085000.htm> | |||
(15) "Biosignal's Furanone Technology." MedGaget. 30 June 2006. 27 Aug. 2008. <http://medgadget.com/archives/2006/06/biosignal_furan.html> | |||
(16) Tiffany, M. J "Tears in health and disease" 5 September 2002. 27 August 2008. <http://www.nature.com/eye/journal/v17/n8/full/6700566a.html> | |||
(17) Walcott, Benjamin "The Lacrimal Gland and Its Veil of Tears" April 1998. 27 August 2008. <http://physiologyonline.physiology.org/cgi/content/full/13/2/97> | |||
(18) The Cornea 27 August 2008. <http://www.tedmontgomery.com/the_eye/cornea.html> | |||
(19) Eyelids and Tear channels 27 August 2008. <http://www.lea-test.fi/en/eyes/lidsncha.html> | |||
(20) "Comparative bacteriology of chronic blepharitis" 1984. 27 August 2008. <http://bjo.bmj.com/cgi/content/abstract/68/8/524> | |||
(21) McCulley, P. James and Ward E. Shine "The lipid layer of tears: dependent on meibomian gland function" 11 September 2003. 27 August 2008. <http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFD-49H6Y6X-1&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=10&md5=cae06adc263756d8bb890e5f40005b89#toc1> | |||
(22) Moreau, M. Judy, et al "Phospholipase A2 in Rabbit Tears: A Host Defense against Staphylococcus aureus" 2001. 27 August 2008. <http://www.iovs.org/cgi/content/abstract/42/10/2347> | |||
(23) Rowland, Belinda. “Pseudomonas Infections.” August 2006. http://www.healthatoz.com/healthatoz/Atoz/common/standard/transform.jsp?requestURI=/healthatoz/Atoz/ency/pseudomonas_infections.jsp | |||
(24) Naradzay, Jerome. “Corneal Ulceration and Ulcerative Keratitis.” November 2006. http://www.emedicine.com/emerg/topic115.htm | |||
(25) W. Gifford, Jones. “Careless Use of Contact Lenses Can Cause Blindness”. March 2003. http://www.canadafreepress.com/medical/ear-nose-throat030203.htm | |||
(26) EK, Mela, et.al. “Ulcerative keratitis in contact lens wearers.” October 2003. http://www.healthatoz.com/healthatoz/Atoz/common/standard/transform.jsp?requestURI=/healthatoz/Atoz/ency/pseudomonas_infections.jsp | |||
(27) H, Zhu, et.al. “Pseudomonas aeruginosa quorum-sensing molecules induce IL-8 production by human corneal epithelial cells.” May 2008. http://www.ncbi.nlm.nih.gov/pubmed/18463485?ordinalpos=6&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum | |||
(28) Lomholt, JA and M Kilian. “Ciprofloxacin susceptibility of Pseudomonas aeruginosa isolates from keratitis.” October 2003. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1920786 | |||
(29) Bitko, Vira, et.al. "Viral Infection of the Lungs through the Eye." January 2007. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1797451 | |||
(30) Spencer, J. F. T. Ragout de Spencer, A. L. (2004). Public Health Microbiology: Methods and Protocols. New Jersey: Humana Press Inc. | |||
(31) Armstrong, R. A. “Bug Eye” Microbiologist, June 2003 http://www.sfam.org.uk/pdf/features/bugeyed.pdf | |||
(32) Kenneth Todar University of Wisconsin-Madison Department of Bacteriology “Staphylococcus”, 2005 http://www.textbookofbacteriology.net/staph.html | |||
(33) Mandell G.L. and Bleck T.P. 1995 ‘Atlas of Infectious Diseases’, Vol. III, 1st Ed. Current Medicine, Philadelphia, USA | |||
(34) Ehrenhaus M. P. “Hordeolum”, 2008 http://www.emedicine.com/oph/TOPIC606.HTM | |||
(35) "eye, human."Encyclopædia Britannica. 2008. Encyclopædia Britannica 2006 Ultimate Reference Suite DVD 5 Apr. 2008 | |||
(36) BioMedia Associates Educational Biology Site: What animal has a more sophisticated eye, Octopus or Insect? | |||
(37) "The Sphenoid Bone." Gray's Anatomy. http://education.yahoo.com/reference/gray/subjects/subject/35#p147 | |||
(38) "The Eye" Accessed August 26,2008 http://academia.hixie.ch/bath/eye/home.html | |||
(39) "Departments. Anterior segment." Cantabrian Institute of Ophthalmology. Accessed August 26,2008. a b "Departments. Anterior segment." Cantabrian Institute of Ophthalmology. | |||
(40) Uzzle, Ted. THE EYE, THE EAR and the Brain. Accessed August 26,2008. http://svconline.com/mag/avinstall_eye_ear_brain/index.html | |||
Edited by | ''Edited by Bennett Vitug, Colleen Lam, Helen Tran, Kevin Bui, Machiko Yamamoto, Philip Ngo and Pan Ching Stacy Lu at University of California-San Diego, students of [mailto:ralarsen@ucsd.edu Rachel Larsen]'' | ||
Latest revision as of 02:58, 20 August 2010
Introduction
The eye, it has been said that the eyes are our window to the world. Of the five senses at our disposal, nothing more than the eyes have greatly infused us with an overwhelming abundance of the stimuli collected from the greater world. The eyes personify as the prevailing instrument that shapes our very individuality. Our perceptions, our interactions, our very definitions of who we are, stem from the visual cues received, processed and reflected back. Hence the eyes have been referred to as the window into our soul.
The evolution of the eyes dates back to the pre-Cambrian explosion when the first proto-eyes emerged. These simplest eyes used to detect light and darkness have evolved into wide ranging complexities and have emerged into myriad forms found today. From the compound eyes found in arthropods with its many facets permitting a wide ranging 360 degree field of vision, to the common single lens eyes that telescope and modulate to generate images, to eyes capable of registering light wavelength in the ultraviolet spectrum, the eyes today are all about the ever increasing information gathering, to provide a better edge and thus enhance survivability. (36)
Our focus today looks not at the anatomy of the eye, but rather as an organ, the eye provide a niche for other organisms to explore, exploit and establish residence within its border. Specifically, we will deal primarily with the human eye and the conditions it provides as the a suitable host. We will also examine some of the defense mechanisms employed by the human body to maintain law and order and thus prevent of the over infestation by undesirable colonization of microorganisms.
The Eye Niche
Physical Location of the Eyes
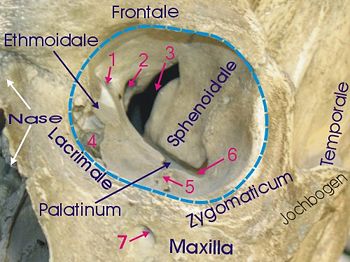
The human eye is located in the anterior portion of the cranium. The eyes are housed within the 7 bones that form the orbit of the eye; the frontal bone, the maxilla, the zygomatic bone, the lacrimal bone, the nasal bone (not part of orbit), the sphenoid bone, the ethmoid bone and the palatine bone. (37)
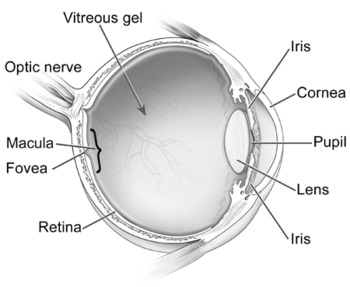
Physical features of the Human Eye
The eye is comprised of three distinct layers. The tunica fibrosa oculi is the outer layer fabricated from the collagen. Its functions include protection of the eye and serve to give the overall round shape of the eyeball. The region also consist of the sclera and cornea. The tunica vasculosa oculi represents the middle layer of the eye. Its vascular region is responsible for the transport of nutrients, wastes, immunoglobulins, and the more important gas exchange of oxygen and carbon dioxide. Amongst the noticeable features of this region is the iris, ciliary body, and the choroid (which are blood vessels responsible for the mentioned functions). The tunica nervosa oculi is the innermost region of the three layers, it is sensory portion of the eye containing rods and cones on the retina. The impulses generated from reactions with light waves are sent to the brain via the optic nerve. (38)
In addition the eye is subdivided into the anterior and posterior chambers. the anterior portion includes the structures in front of the vitreous humour, these are the cornea, the iris, the ciliary body, and the lens. The anterior chamber is bathed in a replenishing aqueous humor. The larger portion is the posterior chamber. These involve the anterior hyaloid membrane and all of the optical structures behind it,the vitreous humor, retina, choroid, and optic nerve. (39)
Conditions of the Eye
Normal pH range in the human eye ranges from 7.0 to 7.4 (very neutral). Normal eyes closed temperature = 34.77′0.37 °C Normal eyes open temperature = 35.02′0.39 °C
The avascular nature of the eye heavily depends on the aqueous humor and the vitreous humor to transport nutrients, wastes, and gas exchanges. Containing the right pH, temperature and proper nutrients, the eye is a great and prized niche for microorganisms.
The aqueous humor is a replenishing solution filled the area between the lens and the cornea. The aqueous humor establishes intraocular pressure of the anterior chamber, resulting in the bulgeoning shape of the eye. In addition, the solution carries nutrition in forms of amino acids and glucose for the avascular ocular tissues namely the posterior cornea, trabecular meshwork, lens, and anterior vitreous. Second, the aqueous humor removes waste products from metabolism of the above avascular ocular tissues. Immunoglobulins provide an immune response to defend against pathogens.
The vitreous humor is a gelatinous mass that occupies the space between the lens of the eye and the retina. The vitreous humor generates the intraocular pressure ranging from 11-21 mm of Hg. The gel in the vitreous chamber is stagnant. Hence there is a high concentration of phagocytes which remove cellular debris impeding the visual field. The vitreous humor contains no blood vessels with 99% of its volume made up of a solution of mostly water and low concentrations of; salts, sugars, vitrosin, as well as a network of collagen. (40)
Conditions under which the environment changes
The eye is a very controlled environment. Thus any changes in chemical concentrations, infiltration of the niche by other microorganisms, or trauma to the eye may affect the natural community of the eye niche. Due to the mechanisms that maintain sterile conditions of the eye, disturbances from microorganism, trauma or chemicals may set off an inflammatory immune responses characterized by mild symptoms such as irritation to more serious problems such as diminishing eye sight.
Defense Mechanisms in the Eye
Barely any microbes live in the eye. This stems from the fact that each section within the eye has mechanisms that defend against infection and microbial colonization.
Blink Reflex
The blink reflex is a mechanical defense against particles in the air or trauma. Eyelashes and the sensitive cornea both participate in this reflex. Tears, debris, allergens, microbes, etc. are moved over to the lacrimal excretory system with the motion from the eyelid.
Barriers
The orbital septum, cornea and conjunctiva all provide a protective barrier against pathogens. The orbit and the eyelid are separated into preseptal and postseptal spaces by the orbital septum which creates a physical barrier against infections. The various layers of the cornea limit permeability of items into the eye. Also, native flora of the lids and mucosal surface limit possible pathogenic colonization. (10)
Tears
Tears provide a mechanical defense via flushing of foreign particles from the surface of the eye and transporting antimicrobial agents to the surface as defensive measures. (11)
Immune Response
The cornea, due to lack of a vascular system, contains limited immune defenses. Immune defenses are provided by Langerhans cells and immunoglobulins. Langerhans cells modify B and T cell in the cornea while glycocalyx (13), a transmembrane mucin made up of a mucin-like glycoprotein adds protection by preventing adhesion of foreign molecules to the surface of the eye.
Langerhans cells detect immunoglobulins, antigen, and complement. They work like macrophages to destroy foreign invaders. The helper B and T cells that are modified by Langerhans cells work in conjunction with lymphocytes to elicit an immune response. (10)
Leukocyte Defense
Leukocytes consume and destroy microorganisms via and oxygen-dependent pathway and an oxygen-independent pathway. The oxygen-dependent pathway uses oxygen radicals while the oxygen-independent pathway utilizes defensins which are peptides that have a broad range of antibacterial, antifungal, and some antiviral activities. (10) (12)
Eyelids
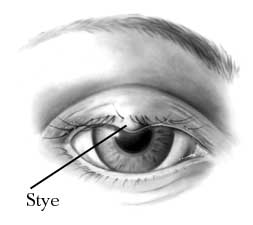
The eyelids are the protective folds to the eyes, which are continuous with the adjacent skin. They consist essentially of an outer sheet of muscle, orbicularis, and an inner fibrous, tarsal plate, covered by skin on the outer side and conjunctiva on the inside.
Although most bacteria found around the eyes usually live harmlessly in the eye niche, they can still cause infections and irritations to the eye. Some of the most common bacterial infections in the eyelid are caused by bacteria from the Staphylococcus family, such as Staphylococcus aureus and Staphylococcus epidermidis. They are harmless under normal conditiona, but they can cause diseases such as blepharitis or hordeola (stye) by entering wounded skin on the eyelids. (31)
Many eye allergies are followed by bacterial infections. Demodex folliculorum is a tiny parasitic mite that lives in or near the hair follicle of mammals and it can cause allergic reaction which leads to the scaling of epidermis, destructions of the eyelash follicles and formation of ulcers. In many cases hordeola follows after blepharitis.
Most people get staph infections by physical contact with contaminated objects. Example such as Hordeolum (stye), is a pustule on the eyelid margins commencing within the follicle of an eyelash or in one of the associated glands caused by presence of Staphylococcus aureus. (34)
Eye cosmetic products are one of many things that can be contaminated with bacteria. They possess nutrients such as lipids, polysaccharides, amino acids, and glycosides...etc, that will facilitate growth of microorganism. With the wrong application method, sometimes the user can break the skin, which leads to staph bacteria entering the wound and causes inflammations and other eye diseases. (30)
Tears
Tears are one of the most important defense systems that our eyes have. Tears are secretion from tear glands that lubricate and protect eyes from infection. The tear film allows gas exchange between the air and the epithelium and also keeps the cornea wet. The substance of the tear film is retinol to provide the transparent nature of the epithelium. (16) Tear fluid is composed of water, electrolytes (sodium and potassium), proteins (immunoglobulin and lysozyme), lipid, mucins, and others such as lactoferrin, lipocalin, and lacritin.
These components of tears help form a defense system that guards the eyes from infection. For example, IgA is the main immunoglobulin of tears and is synthesized by subconjunctival plasma cells. Because of the dimer structure of this protein, it is very effective in aggregating targets to hampers their penetration of the body surface (5). Besides immunoglobulin, another protein called lysozyme has similar functions. “Lysozyme is an enzyme that is naturally abundant in tears and plays an important role in maintaining healthy eyes” (6). Lysozymes are known to have anti-inflammatory and bacteriolytic functions because they can dissolve the outer coating of certain bacteria; and thus, killing the bacteria so that it cannot infect the eye.
In addition, lactoferrin is an iron binding protein secreted by the epithelial acinar cells of the lacrimal glands. Lactoferrin is one of the key molecules that regulate the inflammatory response and the protection against infections. The sequestration of iron by lactoferrin can firmly control bacterial flora and inhibits the iron catalyzed production of hydroxyl radical. Generally, hydroxyl radical may lead to major tissue damage by producing peroxidation of cell membrane lipids, oxidative damage to proteins, and generation of other free radicals. Hence, lactoferrin’s ability to deplete free iron can inhibits the pro-inflammatory effects of hydroxyl radical; and therefore, protects the mucosal surfaces from oxidative damage. Moreover, lactoferrin inhibits the ability of bacteria and viruses to attach to cell membrane of the host cell (7).
Furthermore, lipocalin is another component of tears that form the eye’s defense system. Lipocalin is the major lipid binding protein in tears. Lipocalin can bind to wide variety of lipids such as fatty acids, cholesterol, phospholipids and glycolipids. By interacting with lipids, tear lipocalin can contribute to the surface pressure in the tear film. Moreover, lipocalin also help to promote optical clarity and may transport lipids from the lacrimal gland to the tear film. It plays a significant role in the protection of the ocular surface from desiccation because the lipids that are bounded to the tear lipocalin can serve to provide a reservoir of lipid molecules in equilibrium with the surface and could reduce evaporation of water. Moreover, tear lipocalin can gather lipid from the cornea to prevent dry spots from forming on the cornea (8).
Likewise, lacritin also help promote ocular surface wetting. Lacritin flows onto the ocular surface from the lacrimal and meibomian glands. It is capable of protecting epithelia of the lacrimal-corneal axis against inflammation that associated with cell death.
Tear production system
The protection of tears has three separate layers: the lipid layer, the aqueous layer, and the mucous layer. The outermost lipid layer is a thin layer (0.1-0.2µm that is composed of hydrophobic surface. It contains oils (lipids) produced by the meibomian glands. This oil (lipids) function to retain moisture and prevent tear fluid loss due to evaporation. The temperature in meilbomain gland lipids has the low melting point range 19-32°C depending on the degree of fatty acids unsaturation. The meibomain gland lipids allow lipid get through the meibomian gland ducts and excretion to the tear film.
The aqueous layer is a tear film including water and proteins produced by the lacrimal gland. The middle layer of the lacrimal gland is the thickest (7-8µm) and produces 0.9% saline tears. It functions delivery the main nutrients such as oxygen to the cornea and carrying waste products away from the cornea
The innermost mucous layer (30µm) contains mucin produced by goblet cells. The mucin provides a protective barrier for the surface and serves as a means for the aqueous layer to adhere to the surface of the eye. The mucous’s thin layer of hydrophilic surface can make even on the corneal surface to spread water. (17) (18)(19) and (20)
Conditions which affect the lipid layer or the mucin layer can cause a dry eye.
People with dry eyes lack a complete lipid layer which result in an inadequate amount of tear fluid. Without this tear fluid, the person may experience a burning, gritty sensation and/or blurred vision.
When lipids are restored, fluid is then replenished and moisture is restored in the eyes. (9)
Many species of bacteria are found in the eyelid and conjunctiva as symbiotic relationships. These are Staphylococcus aureus (S. aureus), Staphylococcus epidermidis, Streptococcus, Corynebacterium, Propionibacterium, and Propionibacterium acnes. These bacteria produce lipase that harm to the tear film lipid layer especially, S. epidermidis and S. aureus produce triglyceride lipase and cholesterol and wax esterase. Accumulated cholesterol is used for the growth of certain ocular bacteria. Increased bacteria are regulated by anti-microbial peptides from the lacrimal gland and conjunctiva. (21)
Common bacteria found in Adjacent Communities
Ear: Staphylococcus (coagulase –neg.), Diptheroids, Pseudomonas spp., Enterobacteriaceae spp..
Nose: Staphylococcus (coagulase –neg.), S. aureus, Haemophilus spp., Viridians streptococci, S. pneumoniae
Oropharyn: Staphylococcus (coagulase –neg.), S. aureus, Haemophilus spp., Viridians streptococci, S. pneumonia, Veilonella spp., Prevotella spp., Fusobacterium spp., Candida spp., Moraxella spp., Actinomyces, Eikenella corrodens.
Skin: Staphylococcus (coagulase –neg.), S. aureus, Propionibacterium acnes, Haemophilus spp., Viridians streptococci, Mycobacterium spp., Bacillus spp., Candida spp.
Microscopic Organisms present in the Eye Niche
Bacteria in the Eye Niche
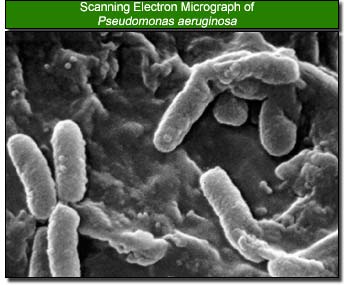
Many normal bacteria are present in the human eye which includes Staphylococcus, Staphylococcus aureus, Haemophilus species, and Streptococcus species. However, many other outside bacteria compete for their stay in the eye niche that may lead to tragic diseases and unfavaorable results.
Bacterial Effects on the Eye Environment via Contact Lens
The most common bacteria that can cause corneal ulcer, which could lead to the development of eye blindness, are staphylococcal species, Pseudomonas aeruginosa, Streptococcus pneumoniae, and Moraxella species. Of these, Pseudomonas aeruginosa is found to be the primary bacterium when wearing contact lens, typically soft contacts, that can cause corneal ulcer. Pseudomonas aeruginosa is a gram-negative, aerobic rod-shaped bacterium which optimally grows at 42 degrees celcius. Although classified as aerobic, it can adapt and live in anaerobic environments. Being an opportunist, this bacterium greatly affects the eye when the defensive mechanisms of the eye are weakened. Otherwise, it does not ordinarily cause disease. It is, however, a very durable bacterium, some of which includes minimal food requirement, a protective outer coating, and resistance to many antibiotics (22).
Dr. Gifford Jones of canadafreepress.com had reported that people who wore contact lens are at risk of developing blinding eye infections during the first six months of use. He proposed that it depended on what the contact lenses were made of. It was said that Pseudomonas aeruginosa infection for soft contact lenses is one in 2,500 and one in 500 if it was worn overnight. For hard contact lenses, infection is one in 10,000. Biodeposits build up on the contact lenses which can cause irritation and corneal ulcers. When contact lenses are worn, it covers up the cornea which restricts oxygen flow to underlying tissues. In an anaerobic environment, Pseudomonas aeruginosa is then able to reside properly. Dr. Dwight Cavanagh, a professor of Opthlalmology at the University of Texas Southern Medical School, stated that new cells on the cornea cannot be produced when the contact lenses cover it, which may decrease the thickness of the cornea by 10 percent (25).
A study from the United Kingdom reported that people who wear soft contact lenses result in 8 times more of a chance for bacteria to invade in the cornea when they sleep than the people who only wear them while they are awake (24).
In 1993, The Department of Ophthalmology at University of Patras medical School in Greece, performed an experiment to determine the clinical microbiological characteristics of corneal ulcers in contact lens wearers. Results showed that, of the ulcerative keratitis patients, 26.74% of them wore contacts. In addition, Pseudomonas aeruginosa was the most frequent isolated bacterium. This experiment concluded that the use of contact lens has a high risk factor for ulcerative keratitis (26).
In addition, a study in Sydney of 2003, Australia found that Pseudomonas aeruginosa produce quorum-sensing signal moleucules which contributed to the induction of the inflammatory response in Pseudomonas keratitis (27).
Resistance to Pseudomonas aeruginosa
Pseudomonas aeruginosa in the eye is targeted by the use of an antibiotic called ciprofloxacin. Current research shows that ciprofloxacin is very effective in treating ulcerative keratitis, although there have been rare results of the bacterium being resistant to the antibiotic, possibly due to the bacterium’s adaptation (28). Pseudomonas aeruginosa has increased its resistance to many antibiotics up to today. Patients who are treats Pseudomonas aeruginosa often have to take multiple antibiotics due to the bacterium’s high barrier of antibiotic resistance (23).
Fungal Infestation of the Eye Niche
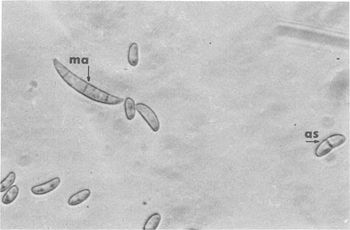
Common fungi found in the Eye: - Acremonium spp. - Aspergillus flavus - Aspergillus fumigatus - Aspergillus niger - Bipolaris spp. - Candida albican - Curvularia spp - Exserohilum spp. - Fusarium oxysporum - Fusarium solani - Lasiodiplodia theobromae
The warm and moist environment of the eye is subject to mycotic infections from fungal species such as Fusarium, Aspergillus, Acremonium, and the Candida species of yeasts. Due to the preferences for moisture-rich environments and warm weather, the prevalence of fungal infections of the eye is higher in tropical areas and in areas with large agrarian communities (3). Despite the relatively sterile environment of the eye, factors such as eye “trauma (generally with plant material), chronic ocular surface diseases, contact lens usage, corneal anesthetic abuse and immunodeficiencies” can result in infection of the eye by filamentous fungi or yeasts (2). Fusarium solani, a species of fungi that is associated with contact lens wearers, normally inhabits warmer climates in soil and organic matter. Invasion of the inner layers of contact lenses and transfer to the surface of the eye, however, is possible due to the ability of Fusarium solani to reside in the moisture-rich inner matrix of soft contact lenses.
Fungal Effects on the Eye Environment
Fungal infections, such as fungal keratitis, can occur on the anterior surface of the eye or the cornea, and may also infect the posterior of the eye as a result of mycosis. Aggregation of fungal microbes into hyphae enlarges the infected region and prevents host inflammatory cells from ingesting the fungi. As a result of the pattern of fungi to join together and form hyphae, fungi that inhabit the eye may extend throughout the entire depth of the cornea, and tend to grow parallel to the lamellae of the cornea (1) (2).
Fungal Metabolism that Affects the Eye Niche
Fungi that inhabit the eye produce proteases that are specific for the surface on which they grow. As a result, protein catabolism by fungal proteases results in tissue damage to the eye, ulceration as well as an inflammatory response around the infected area (1). As a result of catabolic degradation of the eye environment by the fungi, an inflammatory response by the host is accompanied by necrosis of the surrounding eye tissues.
Fungal Infection of the Eye Niche via Contact Lenses
Fungal infection of the eye may occur as a result of fungal growth on contact lenses. Due to tendency of fungi to inhabit moisture-rich locations, contact lens cases and the soft inner matrix of contact lenses are subject to fungal growth. Fungi have the ability to degrade hydrophilic polymers of contact lenses, and may be transferred from contaminated lenses to the surface of the eye, often causing the implantation of fungi into the anterior regions of the eye or the cornea and result in fungal keratitis (1).
Closing Up the Eyes
The eye is an avascular organ bathed in an isotonic solution, which is exposed to the atmosphere and always in contact with microorganisms. Due to the environment and the defense mechanisms of the eye, very few microbes exist in the eye niche. Although the eye possesses natural defense mechanisms such as physical reflexes and immune system response, fungal microbes and bacterial microbes are still capable of infecting the eye. Many infections of the eye can be painful and if left untreated they can lead to more serious damages, so make sure you wash your hands before touching your eyes!
Current Research
1. Iris Pigment Epithelium Expressing CD86 (B7-2) Directly Suppresses T Cell Activation In Vitro via Binding to Cytotoxic T Lymphocyte–associated Antigen 4
A current study at Schepens Eye Research Insitute at Harvard Medical School identified the thin layer of cells lining the iris of the eye, the iris pigment epithelium (IPE), as a tissue that contributes to ocular-immune privilege by secreting immunosuppressive factors and expressing surface molecules that trigger apoptosis by T cells. Scientists exposed PE cells from various regions of the eye to purified T Cells and found that only the IPE cells from the iris responded to CD86 by stopping T cell activity. Normally, T cell activity in the body promotes inflammation. By suppressing its activity, however, IPE cells are able to prevent inflammation in the eye. The results demonstrated that IPE cells contribute to immune privilege and prevent eye inflammation in the iris. The study identified that disruption of IPE cell activity in the eye, perhaps by infection or other factors, may lead to immunogenic inflammation, disruption of the visual axis and blindness (4).
2. Viral Infection of the Lungs through the Eye
Respiratory syncytial virus (RSV) is a virus that is greatly known to affect the respiratory system of newborns. Researchers discovered an association of this virus with allergic conjunctivitis, also known as Pink Eye, which allowed the virus to flow toward the respiratory system initially from the eyes. They experimented on a live mouse by applying RSV to its eyes. RSV not only replicated but also drifted to the lungs, which produced a respiratory disease. Treatment of RSV with anticytokine was successful. They concluded that respiratory infections could be involved with the eye and that it can be treated. (29)
3. Phospholipase A2 in Rabbit Tears: A Host Defense against Staphylococcus aureus
Staphylococcus aureus causes keratitis in many human populations. Tear films have many nutrients that are beneficial for bacteria growth. Tear films also, however, have an effective defense system to reduce replication of bacteria and regulate bacterial growth. One of inhibitors for the host defense molecule is phosholipase A2 (PLA2). Moreau et al. studied the PLA2 effect on Staphylococcal activity. They found PLA2 killed Staphylococcus by hydrolyzing bacterial membranes to release fatty acids and destroyed the bacterial cell wall. (22)
4. Antimicrobial Contact Lenses
Current research on antibacterial contact lenses to prevent microbial colonization into the eye niche, are going through various trials to measure the effectiveness of such lenses in comparison to regular lenses. The lenses were created to address the problem ofmicrobial contamination of lenses ranging from acute red eye to microbial keratitis. The lenses work by "jamming" the signaling systems that bacteria use to form biofilms. (14) The technology involves using a furanone technology to jam the signals and was discovered from a seaweed that generates the anti-biofilm compound. The drug-resistance problem of current antimicrobial agents should not affect these lenses since the technology is not designed to kill bacteria. (15)
References
(1) Wilson LA, Ajello L. Agents of oculomycosis: fungal infections of the eye. In Collier L, Balows A, Sussman M, eds. Microbiology and microbial infections. 9th ed. Vol. 4, London, Arnold, 1998.
(2) Tanure, M. A., E. J. Cohen, S. Sudesh, C. J. Rapuano, and P. R. Laibson. 2000. Spectrum of fungal keratitis at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea. 19:307-12.
(3) L. Alcazar-Fuoli, E. Mellado, A. Alastruey-Izquierdo, M. Cuenca-Estrella, and J. L. Rodriguez-Tudela Aspergillus Section Fumigati: Antifungal Susceptibility Patterns and Sequence-Based Identification Antimicrob. Agents Chemother., April 1, 2008; 52(4): 1244 - 1251.
(4) Sugita, S., J. Streilein. “Iris Pigment Epithelium Expressing CD86 (B7-2) Directly Suppresses T Cell Activation In Vitro via Binding to Cytotoxic T Lymphocyte–associated Antigen 4.” J. Exp. Med., Jul 2003; 198: 161 - 171.
(5) “Biological Functions of Immunoglobulins.” Structure, Function, & Genetics of Immunoglobulins. 20 Aug. 2008. <http://www.lib.mcg.edu/edu/esimmuno/ch3/biologic.htm>
(6) “Lion to Release ‘Smile 40 Mediclear’.” Lion. 30 July 2007. 19 Aug. 2008. <http://www.lion.co.jp/en/press/html/2007017f.htm>
(7) “Tear Lactoferrin.” 20 Aug. 2008. <http://www.touchscientific.com/lfnfact2.htm>
(8) “Dry Eye Disease.” Mission for Vision – Complications of LASIK. 31 Dec. 2005. 19 Aug. 2008. <http://www.missionforvisionusa.org/content/2005/12/dry-eye-disease.html>
(9) “Almera Soothe Eye Drops.” Matheson Optometrists. 25 Aug. 2008. <http://www.matheson-optometrists.com/Products/Almera-Bausch-and-Lomb-Soothe.htm>
(10) Stephen A. Klotz, Christopher C. Penn, Gerald J. Negvesky, and Salim I. Butrus. Fungal and Parasitic Infections of the Eye. Clin Microbiol Rev. 2000 October; 13(4): 662–685. Accessed: 18 Aug 2008. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=88956.
(11) E K Akpek and J D Gottsch. Immune Defense at the Ocular Surface. Eye (2003). 28 Feb. 2003. 17, 949–956. Accessed: 18 Aug 2008. http://www.nature.com/eye/journal/v17/n8/full/6700617a.html
(12) T Ganz, M E Selsted, D Szklarek, S S Harwig, K Daher, D F Bainton, and R I Lehrer. Defensins. Natural peptide antibiotics of human neutrophils. 1985 October; 76(4): 1427–1435. Accessed: 20 Aug 2008. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=2997278.
(13) Maurizio Rolando MD and Manfred Zierhut MD. The Ocular Surface and Tear Film and Their Dysfunction in Dry Eye Disease. 6 Nov. 2001. Accessed: 27 Aug 2008. http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6TCC-44CHKR8-3&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=4429&md5=ecd1d84e68b637836d809a5a8c952bb9.
(14) "First Human Trial of Antibacterial Contact Lenses." ScienceDaily. 29 June 2006. 27 Aug. 2008. <http://www.sciencedaily.com /releases/2006/06/060629085000.htm>
(15) "Biosignal's Furanone Technology." MedGaget. 30 June 2006. 27 Aug. 2008. <http://medgadget.com/archives/2006/06/biosignal_furan.html>
(16) Tiffany, M. J "Tears in health and disease" 5 September 2002. 27 August 2008. <http://www.nature.com/eye/journal/v17/n8/full/6700566a.html>
(17) Walcott, Benjamin "The Lacrimal Gland and Its Veil of Tears" April 1998. 27 August 2008. <http://physiologyonline.physiology.org/cgi/content/full/13/2/97>
(18) The Cornea 27 August 2008. <http://www.tedmontgomery.com/the_eye/cornea.html>
(19) Eyelids and Tear channels 27 August 2008. <http://www.lea-test.fi/en/eyes/lidsncha.html>
(20) "Comparative bacteriology of chronic blepharitis" 1984. 27 August 2008. <http://bjo.bmj.com/cgi/content/abstract/68/8/524>
(21) McCulley, P. James and Ward E. Shine "The lipid layer of tears: dependent on meibomian gland function" 11 September 2003. 27 August 2008. <http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFD-49H6Y6X-1&_user=10&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=10&md5=cae06adc263756d8bb890e5f40005b89#toc1>
(22) Moreau, M. Judy, et al "Phospholipase A2 in Rabbit Tears: A Host Defense against Staphylococcus aureus" 2001. 27 August 2008. <http://www.iovs.org/cgi/content/abstract/42/10/2347>
(23) Rowland, Belinda. “Pseudomonas Infections.” August 2006. http://www.healthatoz.com/healthatoz/Atoz/common/standard/transform.jsp?requestURI=/healthatoz/Atoz/ency/pseudomonas_infections.jsp
(24) Naradzay, Jerome. “Corneal Ulceration and Ulcerative Keratitis.” November 2006. http://www.emedicine.com/emerg/topic115.htm
(25) W. Gifford, Jones. “Careless Use of Contact Lenses Can Cause Blindness”. March 2003. http://www.canadafreepress.com/medical/ear-nose-throat030203.htm
(26) EK, Mela, et.al. “Ulcerative keratitis in contact lens wearers.” October 2003. http://www.healthatoz.com/healthatoz/Atoz/common/standard/transform.jsp?requestURI=/healthatoz/Atoz/ency/pseudomonas_infections.jsp
(27) H, Zhu, et.al. “Pseudomonas aeruginosa quorum-sensing molecules induce IL-8 production by human corneal epithelial cells.” May 2008. http://www.ncbi.nlm.nih.gov/pubmed/18463485?ordinalpos=6&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
(28) Lomholt, JA and M Kilian. “Ciprofloxacin susceptibility of Pseudomonas aeruginosa isolates from keratitis.” October 2003. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1920786
(29) Bitko, Vira, et.al. "Viral Infection of the Lungs through the Eye." January 2007. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=1797451
(30) Spencer, J. F. T. Ragout de Spencer, A. L. (2004). Public Health Microbiology: Methods and Protocols. New Jersey: Humana Press Inc.
(31) Armstrong, R. A. “Bug Eye” Microbiologist, June 2003 http://www.sfam.org.uk/pdf/features/bugeyed.pdf
(32) Kenneth Todar University of Wisconsin-Madison Department of Bacteriology “Staphylococcus”, 2005 http://www.textbookofbacteriology.net/staph.html
(33) Mandell G.L. and Bleck T.P. 1995 ‘Atlas of Infectious Diseases’, Vol. III, 1st Ed. Current Medicine, Philadelphia, USA
(34) Ehrenhaus M. P. “Hordeolum”, 2008 http://www.emedicine.com/oph/TOPIC606.HTM
(35) "eye, human."Encyclopædia Britannica. 2008. Encyclopædia Britannica 2006 Ultimate Reference Suite DVD 5 Apr. 2008
(36) BioMedia Associates Educational Biology Site: What animal has a more sophisticated eye, Octopus or Insect?
(37) "The Sphenoid Bone." Gray's Anatomy. http://education.yahoo.com/reference/gray/subjects/subject/35#p147
(38) "The Eye" Accessed August 26,2008 http://academia.hixie.ch/bath/eye/home.html
(39) "Departments. Anterior segment." Cantabrian Institute of Ophthalmology. Accessed August 26,2008. a b "Departments. Anterior segment." Cantabrian Institute of Ophthalmology.
(40) Uzzle, Ted. THE EYE, THE EAR and the Brain. Accessed August 26,2008. http://svconline.com/mag/avinstall_eye_ear_brain/index.html
Edited by Bennett Vitug, Colleen Lam, Helen Tran, Kevin Bui, Machiko Yamamoto, Philip Ngo and Pan Ching Stacy Lu at University of California-San Diego, students of Rachel Larsen

![Anatomy of the Eye [4].](/images/7/74/Lm.gif)
![Anatomy of the Eye [5].](/images/d/dd/Lr.gif)
