Toothbrush: Difference between revisions
| Line 2: | Line 2: | ||
===Toothbrush as a Niche=== | ===Toothbrush as a Niche=== | ||
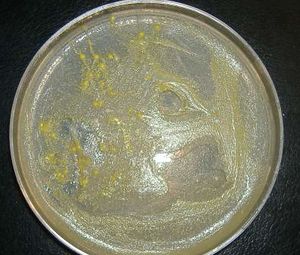
[[Image:ToothbrushBacteria_4DaysGrowth.jpg|thumb| | [[Image:ToothbrushBacteria_4DaysGrowth.jpg|thumb|none|dissection microscope, 10X magnification photograph of unidentified bacteria from a toothbrush used for 9 days and incubated on a nutrient agar plate for 4 days]] | ||
A toothbrush does not naturally contain nutrients to support bacterial growth. However, after brushing, food particles often cling to the bristles of the toothbrush. From being rinsed with water and coming into contact with the moist environment of the mouth, the toothbrush becomes moist. All together, these aspects help make the toothbrush a more favorable environment for microbial growth. The pH of the toothbrush is irrelevant because the microbes are able to survive in a wide range of pH levels.<sup>3</sup> | A toothbrush does not naturally contain nutrients to support bacterial growth. However, after brushing, food particles often cling to the bristles of the toothbrush. From being rinsed with water and coming into contact with the moist environment of the mouth, the toothbrush becomes moist. All together, these aspects help make the toothbrush a more favorable environment for microbial growth. The pH of the toothbrush is irrelevant because the microbes are able to survive in a wide range of pH levels.<sup>3</sup> | ||
Revision as of 07:56, 29 August 2008
The toothbrush is used on a daily basis to clean the oral cavity, so it is a very important piece of equipment known for proper dental hygiene. Sadly, toothbrushes are most commonly located near the bathroom sink, which is a good place to harvest hundreds of microorganisms. No matter how sanitary the bathroom is, the toothbrush will still be consistently exposed to the mouth which will inevitably result in bacterial growth on the toothbrush. A new toothbrush is usually not a favorable habitat for bacteria and fungi, but in some cases, toothbrushes are already slightly infected because there is not a regulation that states toothbrushes must be sold in a sterile package.1 Typically, the presence of microbes on the toothbrush comes from brushing because the mouth is a hospitable niche to many kinds of microbes. Therefore, the bacteria will transfer from the inside of the mouth to the toothbrush.2 In this way, the toothbrush is considered a niche for many microbes.
Toothbrush as a Niche
A toothbrush does not naturally contain nutrients to support bacterial growth. However, after brushing, food particles often cling to the bristles of the toothbrush. From being rinsed with water and coming into contact with the moist environment of the mouth, the toothbrush becomes moist. All together, these aspects help make the toothbrush a more favorable environment for microbial growth. The pH of the toothbrush is irrelevant because the microbes are able to survive in a wide range of pH levels.3
Present microbes
There is a surprising number of microbes that live on a toothbrush, which includes a commonly identified pathogenic fungus known as Candida. In addition, some gram positive pathogenic bacteria such as Streptococcus, Staphylococcus and Lactobacillus, are also present. These microorganisms can potentially cause diseases like gingivitis, pharyngitis, candidiasis, laryngitis, and dental decay if oral hygiene is not well maintained.18
Present non-microbes
Candida albicans is a type of yeast that can be found on a toothbrush. It prefers to live in environments with low pH levels and a lot of moisture. Candida albicans are opportunistic human fungal pathogens that can cause disease in individuals who are immuno-compromised such as HIV/AIDS patients, individuals undergoing chemotherapy or individuals who have had a recent organ transplant.19
Microbe Interaction
Candida albicans are one type of the many organisms that prefer the human mouth as their primary habitat but have abilities that also allow them to survive on the barren surface of a toothbrush. C. albicans can do this by morphing from a free floating or planktonic form to one that is sessile and grows as a community in a biofilm. Biofilms may allow for overpopulation and nutrient load regulation. The formation of a biofilm requires C. albicans to grow hyphae which are multinucleated cell filments that absorb nutrients. They decide to build communities and grow hyphae based on different environmental and cell signals such as an increase in temperature, increase in pH levels, nutrient starvation and increased cell density.20
Not only does Candida form biofilms, but they also incorporate a variety of microbes into the biofilm to benefit one other. A study was done on the effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms to better understand the interactions between multiple species of microbes and their environment. It is found that yeasts can easily re-colonize the oral cavity of an individual who has just undergone antimycotic treatment for oral candidosis, a fungal infection of the mucus membranes in the mouth commonly known as thrush.21
It is observed that when Streptococcus mutans are present, the growth of both Candida albicans and Candida glabrata increases. These results suggest that the microorganisms may co-aggregate in order to enhance the adhesion process in biofilm formation. Not only does S. mutans increase the growth of both Candida species, but they also find a stimulatory effect between C. glabrata and C. albicans when they are co-cultured. By better understanding how these microbes survive in remote sites, such as a toothbrush, oral cavity re-colonization can be avoided by developing better sterilization techniques.22
Survival Rates of Bacteria
Bacteria in mouths can transfer to toothbrushes, so active brushing will cause bacteria to always be present on the toothbrush. Even after the toothbrush is used, the transferred bacteria can survive on it for up to 6 hours at room temperature.4 While on the toothbrush, bacteria have the ability to colonize and multiply in numbers; however, the longer the toothbrush is left to dry, less moisture and nutrients will be available so the concentration of bacteria will begin to decrease.5 The majority of reduction in the concentration of microorganisms happens within 0 to 4 hours after brushing. After more than 4 hours, the decrease in the concentration of microorganisms occurs at a slower rate. When toothpaste is used, brushes show substantially lower numbers of colony-forming units compared to those without the use of toothpaste.6
Influence by Adjacent Communities
Microbes that inhabit a toothbrush must come from somewhere, and a good amount of bacteria on the toothbrush originate from just the mouth, a niche that inhabits a plethora of microbes. The contact of the toothbrush with an oral cavity will thus cause microbes to transfer to the toothbrush.
In addition, a toothbrush's niche can be affected by another toothbrush in close proximity. This is called cross contamination and occurs in a communal environment, since a typical household uses a holder to store multiple toothbrushes. Bacteria living on one toothbrush can thus be transferred to another nearby toothbrush through contact.7 Occurrences like these will spread disease and infect others when using the newly infected toothbrushes. The same idea of bacteria transfer applies when sharing a toothbrush,8 which is a bad hygiene practice.
The toilet also harbors a community of bacteria that can be partially transferred onto the toothbrush. The bacteria is ejected from the toilet bowl after flushing. Results show that during the first two hours after flushing, bacteria are mostly found in a limited area near the toilet. After longer intervals, bacterial colonies are found to be more randomly distributed; the detection of coliform bacteria on surfaces after flushing are also observed. 9
Therefore, it makes sense that individuals should store their toothbrushes in separate holders or locations, preferably away from the toilet as well. The exception is storing a toothbrush in a closed container or cabinet. According to the American Dental Association, dark and moist environments are more favorable towards the growth of microorganisms than open air.10
Conditions That Affect Bacterial Growth
The environments of toothbrushes are usually under normal conditions. The pH of the toothbrush may become more basic because most toothpastes have basic chemical compositions.11 The active ingredient is the reactive fluoride ions which are acidic but their concentrations are negligible compared to other chemical agents in the toothpaste1. The pH of a toothbrush is strictly determined by the chemical residues left behind after brushing which is the toothpaste itself with other minor factors. If the toothbrush is thoroughly washed with tap water, then the pH generally varies from 6-8 which is the pH of tap water. Once the toothbrush has dried, the microbe is no long affected by the environmental pH.
By altering an organism's normal conditions, the survival and growth of the organism can be greatly affected. Toothbrushes have been found to harbor such organisms as Streptococcus mutans12 and Candida albicans13 even days after use. Due to consistent use, moisture and food particles are easily retained by the toothbrush for a certain amount of time, which allows microorganisms to colonize. On a toothbrush, the area most prone to microbial colonization is the area where the bristles are attached to the toothbrush, as well as the area in between bristles.14 Thus, the different ways in which the bristles are attached to the toothbrush and their proximity with one another can have a great effect on the survival and growth of organisms.
Staple-set tufting is a type of bristle attachment that involves taking groups bristles and folding them in half with a piece of metal and then placing them into pre-drilled holes in the toothbrush head. This type of attachment is prone to bacterial colonization because it leaves gaps in the holes between the bristles and the toothbrush, which traps in moisture and food particles.15 A different type of bristle attachment is in-mold placement of bristles, which does not leave any gaps at the base. The bristles are inserted, either in groups or individually, into the toothbrush head and then surrounded at the base with synthetic material. This type of attachment significantly reduces the level of colony forming units; however, it does not completely eliminate bacterial colonization just because there is a lack of gaps at the base.
Another way the organization of bristles effects bacterial growth is how close they are in proximity to nearby bristles. When the bristles of a toothbrush are grouped closely together in tufts, moisture and trapped food particles have a harder time escaping from in between the close-knit bristles; this greatly benefits the bacteria because it gives them the means for survival.16
With these observations, it is evident that by attaching the bristles individually into the toothbrush head so there is a secure seal at the base of the bristles, as well as placing the bristles farther apart instead of in bundles removes any sustenance the bacteria need for survival on the toothbrush. Thus, if changes are made to the environment of the bacteria, such as altering the attachment and proximity of bristles on a toothbrush, the colonization and survival of microbes is significantly reduced.17
Microbes change the environment
One way that C. albicans obtain nutrients on a toothbrush is through a regulatory cascade that controls the secretion of aspartic protease (Saps). C. albicans secrete Saps into their environment to degrade proteins, and they use them as a nitrogen source when there are no other nitrogen sources available such as ammonium or amino acids.23 This may also benefit other microbes in the biofilm by producing nitrogen in the environment that is available to be taken up.
Metabolism
C. albicans and C. glabrata grow in higher numbers in the presence of glucose when compared with sucrose but can survive and grow when either one is available on a toothbrush.24
Current Research
Enter summaries of the most recent research. You may find it more appropriate to include this as a subsection under several of your other sections rather than separately here at the end. You should include at least FOUR topics of research and summarize each in terms of the question being asked, the results so far, and the topics for future study. (more will be expected from larger groups than from smaller groups)
References
Chockalingam, Evvie, and S. Subramanian. “Utility of Eucalyptus Tereticornis (Smith) Bark and Desulfotomaculum Nigrificans for the Remediation of Acid Mine Drainage.” Bioresource Technology 100, no. 2 (January 2009): 615–621. doi:10.1016/j.biortech.2008.07.004.
“Genus Desulfotomaculum - Hierarchy - The Taxonomicon.” Accessed November 5, 2013. http://taxonomicon.taxonomy.nl/TaxonTree.aspx?id=229.
Kaksonen, Anna H., Stefan Spring, Peter Schumann, Reiner M. Kroppenstedt, and Jaakko A. Puhakka. “Desulfotomaculum Thermosubterraneum Sp. Nov., a Thermophilic Sulfate-reducer Isolated from an Underground Mine Located in a Geothermally Active Area.” International Journal of Systematic and Evolutionary Microbiology 56, no. 11 (November 1, 2006): 2603–2608. doi:10.1099/ijs.0.64439-0.
Liu, Yitai, Tim M. Karnauchow, Ken F. Jarrell, David L. Balkwill, Gwendolyn R. Drake, David Ringelberg, Ronald Clarno, and David R. Boone. “Description of Two New Thermophilic Desulfotomaculum Spp., Desulfotomaculum Putei Sp. Nov., from a Deep Terrestrial Subsurface, and Desulfotomaculum Luciae Sp. Nov., from a Hot Spring.” International Journal of Systematic Bacteriology 47, no. 3 (July 1, 1997): 615–621. doi:10.1099/00207713-47-3-615.
Moser, Duane P, Thomas M Gihring, Fred J Brockman, James K Fredrickson, David L Balkwill, Michael E Dollhopf, Barbara Sherwood Lollar, et al. “Desulfotomaculum and Methanobacterium Spp. Dominate a 4- to 5-kilometer-deep Fault.” Applied and Environmental Microbiology 71, no. 12 (December 2005): 8773–8783. doi:10.1128/AEM.71.12.8773-8783.2005.
Ogg, Christopher D, and Bharat K C Patel. “Desulfotomaculum Varum Sp. Nov., a Moderately Thermophilic Sulfate-reducing Bacterium Isolated from a Microbial Mat Colonizing a Great Artesian Basin Bore Well Runoff Channel.” 3 Biotech 1, no. 3 (October 2011): 139–149. doi:10.1007/s13205-011-0017-5.
Pikuta, E, A Lysenko, N Suzina, G Osipov, B Kuznetsov, T Tourova, V Akimenko, and K Laurinavichius. “Desulfotomaculum Alkaliphilum Sp. Nov., a New Alkaliphilic, Moderately Thermophilic, Sulfate-reducing Bacterium.” International Journal of Systematic and Evolutionary Microbiology 50 Pt 1 (January 2000): 25–33.
1. Glass, R., and M. Lare. "Toothbrush contamination: a potential health risk?" Quintessence Int 17 (1986): 39-42.
2. Kozai, K., T. Iwai, and K. Miura. "Residual contamination of toothbrushes by microorganisms." Journal of Dentistry for Children 56 (1989): 210-14.
3. Bowden, George, and Ian Hamilton. "Survival of Oral Bacteria." Critical Reviews in Biology and Medicine 9 (1998): 54-85.
4. Kozai, K., T. Iwai, and K. Miura. "Residual contamination of toothbrushes by microorganisms." Journal of Dentistry for Children 56 (1989): 210-14.
5. Wetzel, Willi-Eckhard, Caroline Schaumburg, Franziska Ansari, Torsten Kroeger, and Andreas Sziegoleit. "Microbial contamination of toothbrushes with different principles of filament anchoring." The Journal of the American Dental Association 136 (2005): 758-65.
6. Efstratiou, M., W. Papaioannou, M. Nakou, E. Ktenas, I. Vrotsos, and V. Panis. "Contamination of a toothbrush with antibacterial properties by oral microorganisms." Journal of Dentistry 35 (2007): 331-37.
7. Neal, Peter, and John Rippin. "The efficacy of a toothbrush disinfectant spray—an in vitro study." Journal of Dentistry 31 (2003): 153-57.
8. Neal, Peter, and John Rippin. "The efficacy of a toothbrush disinfectant spray—an in vitro study." Journal of Dentistry 31 (2003): 153-57.
9. Gerba, Charles, Craig Wallis, and Joseph Melnick. "Microbiological Hazards of Household Toilets: Droplet Production and the Fate of Residual Organisms." Applied Microbiology 30 (1975): 229-37.
10. Council on Scientific Affairs, compiled. "ADA Statement on Toothbrush Care: Cleaning, Storage and Replacement." American Dental Association. Nov. 2005. American Dental Association.
11.
12. Svanberg, M. "Contamination of toothpaste and toothbrush by Streptococcus mutans." European Journal of Oral Sciences 86 (1978): 412-14.
13. Wetzel, Willi-Eckhard, Caroline Schaumburg, Franziska Ansari, Torsten Kroeger, and Andreas Sziegoleit. "Microbial contamination of toothbrushes with different principles of filament anchoring." The Journal of the American Dental Association 136 (2005): 758-65.
14. Wetzel, Willi-Eckhard, Caroline Schaumburg, Franziska Ansari, Torsten Kroeger, and Andreas Sziegoleit. "Microbial contamination of toothbrushes with different principles of filament anchoring." The Journal of the American Dental Association 136 (2005): 758-65.
15. Wetzel, Willi-Eckhard, Caroline Schaumburg, Franziska Ansari, Torsten Kroeger, and Andreas Sziegoleit. "Microbial contamination of toothbrushes with different principles of filament anchoring." The Journal of the American Dental Association 136 (2005): 758-65.
16. Wetzel, Willi-Eckhard, Caroline Schaumburg, Franziska Ansari, Torsten Kroeger, and Andreas Sziegoleit. "Microbial contamination of toothbrushes with different principles of filament anchoring." The Journal of the American Dental Association 136 (2005): 758-65.
17. Wetzel, Willi-Eckhard, Caroline Schaumburg, Franziska Ansari, Torsten Kroeger, and Andreas Sziegoleit. "Microbial contamination of toothbrushes with different principles of filament anchoring." The Journal of the American Dental Association 136 (2005): 758-65.
18.
19. Devine, D. "Inhibition of biofilms associated with dentures and toothbrushes by tetrasodium EDTA." Journal of applied microbiology 103.6 (2007):2516-2524.
20. Martins, M. "Morphogenesis control in Candida albicans and Candida dubliniensis through signaling molecules produced by planktonic and biofilm cells." Eukaryotic cell 6.12 (2007):2429-2436.
Edited by [James Lin, Natalie Nguyen, Nicholas Phung, Sarah Fernandes], students of Rachel Larsen