Canine Distemper Virus: Wildlife Conservation Implications: Difference between revisions
| Line 69: | Line 69: | ||
As more and more species are pushed to the brink of extinction, conservationists are increasingly relying on ''ex situ'' conservation strategies such as captive breeding programs as last-ditch efforts to save species. Captive breeding programs can be used to establish new populations of endangered species, bolster existing ones, and preserve species' genetic diversity. However, CDV not only impedes ''in situ'' conservation efforts, but has devastated various captive breeding programs. | As more and more species are pushed to the brink of extinction, conservationists are increasingly relying on ''ex situ'' conservation strategies such as captive breeding programs as last-ditch efforts to save species. Captive breeding programs can be used to establish new populations of endangered species, bolster existing ones, and preserve species' genetic diversity <ref name=Fraser> [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3352391/] </ref>. However, CDV not only impedes ''in situ'' conservation efforts, but has devastated various captive breeding programs. | ||
Revision as of 03:09, 12 April 2018
By Sam Lisak
Baltimore Classification
Group V: (−) sense single-stranded RNA viruse
Higher Order Taxa
Order: Mononegavirales
Family: Paramyxoviridae
Genus: Morbillivirus
Species: Canine Distemper Virus
Description and Significance
Canine distemper virus (CDV) is a viral pathogen that is commonly known for its ability to infect domestic dogs, though it affects many other species. This single-stranded, negative-sense RNA virus belongs to the Paramyxoviridae family and the genus Morbillivirus [1]. CDV is highly contagious, spreading through aerosol droplets and bodily fluids. Generally, the virus initially replicates in the respiratory tract where it enters the blood stream and spreads to lymphoid, nervous, and epithelial tissues [2]. While symptoms depend on the host species as well as the strain and severity of the virus, CDV often causes fever, ocular discharge, lethargy, anorexia, vomiting, diarrhea, seizure, paralysis, and the thickening of feet pads [3]. Although much research on this virus concentrates on its relationship to domestic dogs, CDV affects many carnivore families such as Canidae (domestic and wild canids), Ursidae (bears), Pinnipeds (seals, sea lions, etc.), and Felidae (wild cats), to name a few [4]. Due to the wide range of potential hosts and the easy transmission pathway from domestic dogs to wildlife, CDV poses a significant threat to the conservation efforts of many threatened mammals. This report attempts to summarize current research on CDV as well as provide insight on how the virus affects wildlife, particularly, the conservation of threatened species.
Virion and Genome Structure
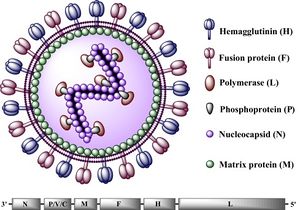
All Morbilliviruses, including CDV, are virions enveloped by a lipid bilayer containing a single-stranded, negative-sense RNA genome [6]. CDV virus particles typically are between 150-300 nm in diameter [7].Their genomes code for six structural proteins in the following order: N (nucleocapsid), P
(phosphoprotein). M (matrix protein). F (fusion protein) (See Figure 2).
H (hemagglutinin protein), and L (large protein). They also code for V and C, two non-structural proteins [5]. The P and L proteins are coupled and attached to the N protein, which surrounds the genome to form the nucleocapsid. The P protein serves as the co-factor for the viral RNA-dependent RNA polymerase (L protein) [5]. The two glycoproteins, H and F, are spikes embedded in the lipid bilayer that mediate fusion between other cells. The envelope-associated M protein is evenly arranged underneath the lipid bilayer and regulates the two glycoproteins in addition to linking the nucleoplasmid with these proteins [5].
The CDV genome consists of 15,616 nucleotides and parallels the genomes of other morbilliviruses, particularly that of its closest relative, the measles virus (MV) [8]. This genome begins with the putative regulatory 3' leader (55 nucleotides long) that directly precedes the N gene, which is then followed by the P, M, F, H, and L genes. Between the L gene and the end of the CDV genome (the 5' terminus), lies the putative 5' leader region (38 nucleotides long) [9]. The putative sequences direct the transcription and replication of the genome. Intergenic sequences, located at the 3' and 5' ends of every structural gene segment control transcription termination and reinitiation [10].
Viral Ecology and Pathology
Include some current research in each topic, with at least one figure showing data.
Signs and Symptoms
Prevention and Treatment

The prevention of CDV, while simple in theory, is almost impossible to successfully implement. Effective vaccines exist and are the only realistic solution for controlling canine distemper in domestic dogs [13] (See Figure 5). While CDV vaccines have greatly decreased the prevalence on canine distemper, the disease still spreads among unvaccinated populations including animal shelters, pet stores, and feral dogs. Additionally, many domestic dogs are suceptible to CDV if their owner forgets or declines to vaccinate their puppies with the required booster shots that are needed until the puppy reaches 16 weeks [14]. Prevention of CDV in captive wild animals can be achieved through careful vaccination, however these vaccines must be proven to be safe for the animals as they can occasionally have adverse effects [13]. However, the widespread vaccination of wild animals is obviously unfeasible, so effective conservation and wildlife management strategies must be implemented to achieve prevention (possible solutions described below).
Despite the widespread prevalence of CDV vaccinations, there are currently no antiviral drugs or specific treatments for canine distemper [15]. Available treatments are symptomatic, aimed at controlling neurological dysfunction, maintaining fluid balance, and defend against secondary bacterial invasions [16]. However, there is hope for an antiviral medication after studies have shown the drugs ribavarin (RBV) and interferon-alpha (IFNα) inhibit the replication of CDV in vitro [15].
Effect on Wildlife Conservation
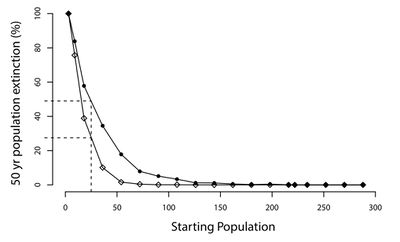
The ability of CDV to infect all terrestrial carnivore families (Mustelidae, Ursidae, Viverridae, Procyonidae, Felidae, Hyaenidae, and Canidae) allows it to threaten the conservation efforts and long-term survival of many species [18] [19]. Large predators are frequently in danger of extinction due to their reliance on large tracts of habitat, vulnerability to poaching, and frequent conflicts with humans. Exposure to CDV only exacerbates the pressure on these struggling populations of carnivores. Part of what makes CDV so dangerous to wildlife populations is its ability to spread between many species [20]. Domestic dogs are most common reservoir for the transmission of CDV to wildlife populations. As the human population continues to expand, the threat of the transfer of CDV to wildlife populations only grows [21].
As more and more species are pushed to the brink of extinction, conservationists are increasingly relying on ex situ conservation strategies such as captive breeding programs as last-ditch efforts to save species. Captive breeding programs can be used to establish new populations of endangered species, bolster existing ones, and preserve species' genetic diversity [22]. However, CDV not only impedes in situ conservation efforts, but has devastated various captive breeding programs.
CDV gained national attention when it nearly drove the black-footed ferret (Mustela nigripes) to extinction.
Potential Conservation Solutions

References
Edited by student of Joan Slonczewski for BIOL 238 Microbiology, 2018, Kenyon College.
- ↑ Frisk, A.L., König, M., Moritz, A., Baumgärtner, W. (1999). Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. Journal of Clinical Microbiology, 37(11), 3634-3643.
- ↑ Expression of canine distemper virus receptor nectin-4 in the central nervous system of dogs. Scientific Reports, 7(349), 1-9. doi: 10.1038/s41598-017-00375-6
- ↑ V. Martella, F. Cirone, G. Elia, E. Lorusso, N. Decaro, M. Campolo, C. Desario, M.S. Lucente, A.L. Bellacicco, M. Blixenkrone-Møller, L.E. Carmichael, C. Buonavoglia. (2006). Heterogeneity within the hemagglutinin genes of canine distemper virus (CDV) strains detected in Italy. Veterinary Microbiology, 116(4), 301-309. doi: 10.1016/j.vetmic.2006.04.019
- ↑ Yuan, C., Liu, W., Wang, Y., Hou, J., Zhang, L., & Wang, G. (2017). Homologous recombination is a force in the evolution of canine distemper virus. PLoS ONE, 12(4), 1-16. doi: 10.1371/journal.pone.0175416
- ↑ 5.0 5.1 5.2 5.3 [1]
- ↑ [2]
- ↑ [3]
- ↑ [4]
- ↑ [5]
- ↑ [6]
- ↑ [7]
- ↑ [8]
- ↑ 13.0 13.1 [9]
- ↑ [10]
- ↑ 15.0 15.1 [11]
- ↑ [12]
- ↑ [13]
- ↑ [14]
- ↑ [15]
- ↑ [16]
- ↑ [17]
- ↑ [18]
- ↑ [19]
