Oil spills: Difference between revisions
| Line 34: | Line 34: | ||
===Microbial community composition=== | ===Microbial community composition=== | ||
A very diverse group and microbes have been showed to have the ability to degrade petroleum hydrocarbons (Atlas 1981, 180-209), among which bacteria and fungi appear to be the prevalent hydrocarbon degraders in oil contaminated ecosystems. | A very diverse group and microbes have been showed to have the ability to degrade petroleum hydrocarbons (Atlas 1981, 180-209), among which bacteria and fungi appear to be the prevalent hydrocarbon degraders in oil contaminated ecosystems. | ||
====Bacteria and archaea==== | ====Bacteria and archaea==== | ||
[[Image: alkane bacteria.jpg|300px|thumb|right|[http://www.scientificamerican.com/slideshow.cfm?id=gulf-oil-eating-microbes-slide-show <i>Alcanivorax borkumensis</i> is a rod-shaped bacteria that uses oil hydrocarbons as its exclusive source of carbon and energy. Although barely detectable in unpolluted water body, it blooms right after an oil spill. It was found in oil spills from Alaska (Exxon Valdez) to the Mediterranean waters near Spain (Prestige). It has specific ability to both break down the alkanes that make up part of the oil, and spread a biosurfactant that helps make oil more bioavailable to other microbes. These unique combination of these features provides this microbe with a competitive edge in oil-polluted environment and therefore it was of special interest of many scientists.]]] | |||
Many bacterial strains have been reported to have the ability to degrade recalcitrant compounds in petroleum. More than 20 genera of hydrocarbon degrading bacteria has been isolated including Alpha-, Beta- and <i>[http://en.wikipedia.org/wiki/Gammaproteobacteria Gammaproteobacteria]</i>; [http://en.wikipedia.org/wiki/Gram-positive Gram positives]; <i>Flexibacter–Cytophaga–Bacteroides</i> (Teralmoto et al. 2009). On the other hand, fast development of molecular microbiological tool has enabled the identification of many un-culturable microbes and therefore extended the list of microbial species with petroleum hydrocarbon degrading abilities. Species of <i>[http://microbewiki.kenyon.edu/index.php/Pseudomonas Pseudomonas]</i>, <i>[http://microbewiki.kenyon.edu/index.php/Mycobacterium Mycobacterium]</i>, <i>[http://en.wikipedia.org/wiki/Haemophilus Haemophilus]</i>, <i>[http://microbewiki.kenyon.edu/index.php/Rhodococcus Rhodococcus]</i>, <i>[http://en.wikipedia.org/wiki/Paenibacillus Paenibacillus]</i> and <i>[http://en.wikipedia.org/wiki/Ralstonia Ralstonia]</i>, are some of the most extensively studied bacteria for their bioremediation capability. | Many bacterial strains have been reported to have the ability to degrade recalcitrant compounds in petroleum. More than 20 genera of hydrocarbon degrading bacteria has been isolated including Alpha-, Beta- and <i>[http://en.wikipedia.org/wiki/Gammaproteobacteria Gammaproteobacteria]</i>; [http://en.wikipedia.org/wiki/Gram-positive Gram positives]; <i>Flexibacter–Cytophaga–Bacteroides</i> (Teralmoto et al. 2009). On the other hand, fast development of molecular microbiological tool has enabled the identification of many un-culturable microbes and therefore extended the list of microbial species with petroleum hydrocarbon degrading abilities. Species of <i>[http://microbewiki.kenyon.edu/index.php/Pseudomonas Pseudomonas]</i>, <i>[http://microbewiki.kenyon.edu/index.php/Mycobacterium Mycobacterium]</i>, <i>[http://en.wikipedia.org/wiki/Haemophilus Haemophilus]</i>, <i>[http://microbewiki.kenyon.edu/index.php/Rhodococcus Rhodococcus]</i>, <i>[http://en.wikipedia.org/wiki/Paenibacillus Paenibacillus]</i> and <i>[http://en.wikipedia.org/wiki/Ralstonia Ralstonia]</i>, are some of the most extensively studied bacteria for their bioremediation capability. | ||
| Line 44: | Line 47: | ||
====Fungi==== | ====Fungi==== | ||
Revision as of 21:15, 21 April 2011
Introduction
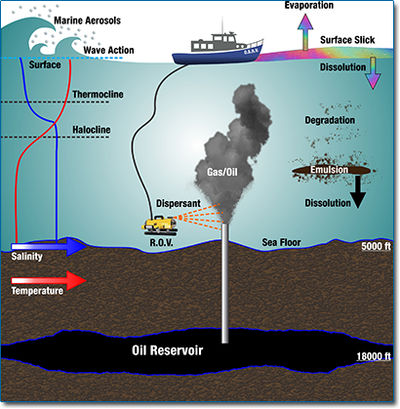
As the demand for crude oil as energy source is fast increasing, accidental and associated oil spillhave been more and more frequent during the process of exploration, production, transportation and storage. On land, oil spills are usually localized and the impact is limited in a certain area. However, when oil spills happens in marine system it may result in very serious ecological risks and long-term environmental disturbance (Patin 2004). The primary source of oil input into seas is usually associated with transportation by tankers and pipelines.
Once an oil spill happens, the oil will experience a series of physical, chemical and biological change, the effect of which is overall defined as “weathering”. However, this process happens slowly in the natural environment and requires a long time for the contaminated environment to recover. Various remediation methods have been proposed, among which the biological remediation has drawn increasing attention recently. There are two general approaches: to apply fertilizers or surfactants in order to promote the microbial degradation process, and to add naturally occurring adapted microbial hydrocarbon degraders seeding (Mulkinsphillips and Stewart 1974). The rate of biodegradation in a remediation site is highly dependent on the characteristics of spilled oil, the localized physical chemical environment, as well as the indigenous microbe’s communities. In order to better use bioremediation, lots of research are being conducted, focusing on both the biotic and abiotic factors influencing the degradation rate.
Physical environment
Oil spill can happen everywhere, from arctic to tropical marine, from soil to water, and therefore the physical and chemical environment of oil spill can vary significantly from one to other. Many factors have been proved to influence microbial degradation of petroleum, including physical status of oil spill, temperature, oxygen, nutrients, soil type and characteristics, and vegetation etc.
Physical status of oil spill
Physical status of oil spill is the most important factor determines the environment the petroleum degraders live. The status of oil spill and those environmental factors will have complex interactions and exert mark influence on microbial communities. Generally, there are two kinds of oil spills: Oil spill in water and oil spill in soil. The biggest different between them is the degree of spread. In soil, hydrocarbon is absorbed by plant and soil particles, limiting its spreading. Oil on soil surface will receive relatively ample oxygen which is crucial for biodegradation. Some portion will go deeper into the soil, but still within limited depth. In that situation, when oxygen becomes limiting factor, aeration is needed in order to enhance the bioremediation (Prince 1993).
In marine environment, there can be a lot of forms of oil; each has its own typical environment. Most of the oil normally spreads, forming a thin slick on the top of water body. A large portion of the oil also forms emulsions or dissolves in the water. Some heavy portions will settle into to the bottom. Oxygen can be relatively easily obtained in the slick oil spill, but the nutrients are sometimes limited. The dissolved oil or oil emulsion droplets usually have chance to obtain both oxygen and nutrient when they travel through water column. The environment in the sediments is low in oxygen although the nutrient may not be limited. The difference in these environmental factors can create a big difference in the petroleum degradation process. For example, it was found that the petroleum hydrocarbons would persist for longer periods in reduced sediments than would it do in aerated surface oil layers (Hamnrick et al. 1980). In addition, the toxic concentration, which is mostly caused by aromatic hydrocarbons, will vary within several orders of magnitude among different microbes. The toxicity has been proven to be crucial factor for biodegradation of petroleum hydrocarbon.
Temperature
The influence of temperature on biodegradation are interactive with other factors, such as the quality of the hydrocarbon mixtures and the composition of the microbial community. First, the temperature affects the physical status of petroleum. Higher temperature will result in higher viscosity, the volatilization of toxic alkanes is reduced, and their water solubility increased, delaying the rate of biodegradations. The second effect is on microbial activity. Higher temperature usually resulted in better activity of enzymes and therefore increased rate of metabolism. Actually, biodegradation activity has been found in almost all environments with extreme temperatures. Biodegradation can occur at very low temperature, e.g. 5 ºC even in arctic zone (Mulkinsphillips and Stewart 1974), and tropical area(Teralmoto et al. 2009). In addition, temperature will affect other environmental parameters that might be crucial to petroleum biodegrading microbes, for example, the availability of liquid water. Atlas (1978) found that the petroleum hydrocarbons from Prudhoe Bay crude oil in Arctic marine’s ice ecosystems were degraded extremely slowly mainly because of the lack of liquid water rather than the temperature itself.
Nutrients
Microorganisms require nitrogen and phosphorus for decomposition of hydrocarbons and incorporation into biomass, therefore the availability of these nutrients are important for bioremediation. Still, the nutrient availability depends heavily on the physical status of oil spill, whether it is in soil or marine, whether it is in dissolved forms or slick. However, in most studies, there seems to be insufficient nutrients to carry out best degradation performance. Many researchers have reported that the amendment of nutrient can significantly improve the degradation of petroleum pollutants both in water and soil (Xu and Obbard 2004, Liang et al. 2011; Tyagi et al. 2011)
Oxygen
The major degradation pathways for petroleum hydrocarbons involves oxygenates and molecular oxygen, indicating the (Boufadel et al. 2010) importance of oxygen for oil degradation microbes. There are anaerobes capable of degradation, but the rate is significantly lower. For instance, within anoxic basins or sediments, the hypolimnion of stratified lakes, and benthic sediments, oxygen may severely limit biodegradation. Oxygen consumption by oil-degrading bacteria is high, even in some environment such as the water surface where oxygen is readily available right after the oil spill happens, oxygen may rapidly become limiting factor due to slow oxygen diffusion rates and its limited solubility in water. Since most of the degradation is aerobic processes, maintenance of aerobic conditions is important in the bioremediation (Atlas 1991)
Salinity and pressure
The influence of several other environmental factors on hydrocarbon biodegradation has been studied. Typically these factors are associated with the localized environment features such as saline lakes or deep seas where high hydrostatic pressure exists. Studies have shown that generally the biodegradation rate decreases as salinity increases. The deep benthic zone in marine ecosystems has been found to be one of those with least microbial activity, partly due to the higher pressure that limits the microbial activity (Shin and Pardue 2001).
Microbial communities
Microbial community composition
A very diverse group and microbes have been showed to have the ability to degrade petroleum hydrocarbons (Atlas 1981, 180-209), among which bacteria and fungi appear to be the prevalent hydrocarbon degraders in oil contaminated ecosystems.
Bacteria and archaea
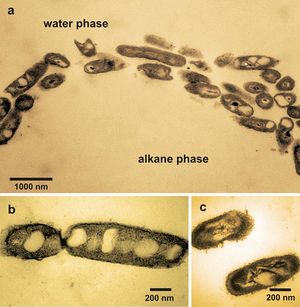
Many bacterial strains have been reported to have the ability to degrade recalcitrant compounds in petroleum. More than 20 genera of hydrocarbon degrading bacteria has been isolated including Alpha-, Beta- and Gammaproteobacteria; Gram positives; Flexibacter–Cytophaga–Bacteroides (Teralmoto et al. 2009). On the other hand, fast development of molecular microbiological tool has enabled the identification of many un-culturable microbes and therefore extended the list of microbial species with petroleum hydrocarbon degrading abilities. Species of Pseudomonas, Mycobacterium, Haemophilus, Rhodococcus, Paenibacillus and Ralstonia, are some of the most extensively studied bacteria for their bioremediation capability.
Archaea have been detected in several oil-containing environments, such as petroleum reservoirs, underground crude oil storage cavities, and hydrocarbon-polluted aquifers. Archaea have also been found in oil contaminated environment degrading petroleum hydrocarbons. Lots of Archaea species are found in oil reservoirs, such as Thermococcus celer, Pyrococcus lithotrophicus, Archaeoglobus fulgidus (Ollivier et al. 2000;de Brito et al. 2004).
As the molecular biology are developing, scientists and engineers are probing more and more into the mechanisms and genetic traits of these oil-eaters, and are expecting to better utilize them to clean up oil spill faster and more efficient. Pseudomonas species is one of the most common species that have been isolated from oil spill bacteria. The genetic information for hydrocarbon degradation has been found to occur on plasmids (Vila et al. 2010) and this genetic trait shows potential to be transferred to other microbes to make some potential “supermicrobes”. Another interesting bacterium, Alcanivorax borkumensis, as shown in the Figure on the right, is the first carbonnoclastic bacterium that has been sequenced (Santos et al. 2006) in order to get valuable insights into its unusual metabolic capability in oil hydrocarbon degradation.
Fungi
Fungi, including yeast and filamentous fungi have also been found to have the ability to degrade petroleum carbon. Species reported include Candida, Rhodotorula, Saccharomyces, Sporobolomyces, and Trichosporon etc. (Liu et al. 2009; Atlas 1981).
Algae and cyanobacteria
Interestingly, the ability to oxidize petroleum hydrocarbons is widely distributed, not only among bacteria and fungi, but also among the cyanobacteria and algae. Cerniglia et al. found that the ability to oxidize aromatic hydrocarbons is widely distributed among algae and cyanobacteria. They tested the capability of naphthalene degradation among nine cyanobacteria, five green algae, one red alga, one brown alga, and two diatoms. Results showed that Oscillatoria spp., Microcoleus sp., Anabaena spp., Agmenellum sp., Cylindretheca sp.,Coccochloris sp., Ulva sp., Nostoc sp., Aphanocapsa sp., Chlorella spp., Dunaliella sp., Chlamydomonas sp., Amphora sp., Porphyridium sp., and Petalonia are all capable of oxidizing naphthalene(Gibson et al. 1980). Except various low molecular weight organics, algae also showed ability to degrade high molecular weight PAHs, such as benzo[a]pyrene(Naidu and Juhasz 2000). Beside single compounds, some researchers also investigated the algae/cyanobacteria capability of degrading the whole matrix of oil. For example, Gamila and Ibrahim (Ibrahim and Gamila 2004) valuate the potential role of freshwater isolated algae strains in biodegradation of crude oil (Egyptian light crude oil, specific gravity 0.85) though an algal bioassay. They found that the diatom strain, Nitzschia linearis, and green alga Scenedesmus obliquus treated with 0.1% crude oil showed almost similar capability for degradation of the petroleum hydrocarbons. Interestingly, they also observed the effect of the crude oil on the morphological characters of these two algae. The most pronounced feature is that the algal cells in both strains were aggregated in clusters containing oil drops between their cells, forming an abnormal shape comparing with control culture.
Microbial community dynamics
Both laboratory and field studies have shown that hydrocarbon contamination shifts the overall microbial community structure (Cerniglia et al.1980). Once the site is contaminated, the microbial community composition will be greatly changed both in quantity and composition. Populations of hydrocarbon-degraders normally constitute less than 1% of the total microbial communities, but when oil pollutants are present these hydrocarbon-degrading populations increase, Certain hydrocarbon-degrading taxa become dominant in oil-impacted environments because of natural selection resulting from the pressure of oil contaminants typically to 10% of the community, or even 100%(Tyagi et al. 2011).
Microbial processes
Aerobic processes
Oil is a complex mixture of various components, with different affinity with microbes. The biodegradability of the oil components generally decreases in the following order: n-alkanes, branched-chain alkanes, branched alkenes, low molecular-weight n-alkyl aromatics, monoaromatics, cyclic alkanes, polycyclic aromatic hydrocarbons (PAHs) and asphaltenes (Atlas 1981). No matter what form the component is, these compounds are eventually converted to carbon dioxide and water by microorganisms if in aerobic degradation process, the most common biodegradation.
The initial steps in the aerobic biodegradation by bacteria and fungi involve the oxidation of the substrate by oxygenases for which molecular oxygen is required. Aerobic conditions are, therefore, necessary for microbial oxidation of hydrocarbons in various environments. The availability of oxygen in soils, sediments, and aquifers is often limiting and dependent on the type of soil, whether the soil is aerated, weather the water is well mixed etc.
Anaerobic processes
Anaerobic degradation exists, however, the rate of which is very low and the ecological significance appears to be minor. Anaerobic respiration of oil hydrocarbons can be found in the sediments or anoxic zones, where Mn+2, Fe+2 can be the terminal electron acceptor (Atlas 1991; Essaid 1995).
Current Research
1. In order to compare microbial functional diversity in different oil contaminated soils, and to find the relationship between the contamination and environmental factors, Liang et al. analyses soil samples from 5 different oil filed and used GeoChip to evaluate the microbial functional genes. Results showed that the samples were clustered by geographic locations and the contaminant degradations genes presented similar patterns under oil contaminant stress. Canonical analysis results also indicated that the local environmental variables significantly affect the microbial functional patterns (Boufadel et al. 2010).
2. In order to evaluate the long-term environmental effect of Exxon Valdez oil spill in 1989, a series of measurements of the background concentration of nutrients, dissolved oxygen (DO), and salinity were obtained from a contaminated beach. Results showed that both nutrients and DO are limiting factors for biodegradation. Also, the lowest nitrate and DO values were found in the oiled pits, implying that the microbial oil degradation was probably under anoxic conditions associated with denitrification (Liang et al. 2011; Vila et al. 2010).
3. The feasibility of a bioaugmentation strategy based on use of microbial formula tailored with selected native strains to remediate diesel contaminated site was assessed. The biodegradation process of diesel oil was assessed by monitoring the DO composition, CO2 evolution rate, microbial load and composition of the community by T-RFLP, physiological profile in Biolog® ECOplates and ecotoxicity. The formula—ENEA-LAM that combines 10 bacterial strains selected for resistance to heavy metals was found to efficiently facilated and speed up the bioremediation of diesel hydrocarbons and heavey metals (Boufadel et al. 2010) .
References
Alisi, Chiara, Rosario Musella, Flavia Tasso, Carla Ubaldi, Sonia Manzo, Carlo Cremisini, and Anna Rosa Sprocati. 2009. Bioremediation of diesel oil in a co-contaminated soil by bioaugmentation with a microbial formula tailored with native strains selected for heavy metals resistance. Science of the Total Environment 407 (8) (4/1): 3024-32.
Atlas, R. 1981. Microbial-degradation of petroleum-hydrocarbons-an environmental perspective. Microbiological Reviews 45 (1): 180-209.
Atlas, R., A. Horowitz, and M. Busdosh. 1978. Prudhoe crude-oil in arctic marine ice, water, and sediment ecosystems-degradation and interactions with microbial and benthic communities. Journal of the Fisheries Research Board of Canada 35 (5): 585-90.
Atlas, Ronald M. 1991. Microbial hydrocarbon degradation-bioremediation of oil spills. Journal of Chemical Technology & Biotechnology 52 (2): 149-56.
Boufadel, M., Y. Sharifi, B. Van Aken, B. Wrenn, and K. Lee. 2010. Nutrient and oxygen concentrations within the sediments of an alaskan beach polluted with the exxon valdez oil spill. Environmental Science Technology 44 (19): 7418-24.
Cerniglia, C. E., D. T. Gibson, and C. Vanbaalen. 1980. Oxidation of naphthalene by cyanobacteria and microalgae. Journal of General Microbiology 116 (FEB): 495-500.
Essaid, H. I. 1995. Simulation of aerobic and anaerobic biodegradation processes at a crude oil spill site. Water Resources Research 31 (12): 3309.
Hamnrick, G., R. Delaune, and W. Patrick. 1980. Effect of estuarine sediment pH and oxidation-reduction potential on microbial hydrocarbon degradation. Applied and Environmental Microbiology 40 (2): 365-9.
Liang, Y., J. D. Van Nostrand, Y. Deng, Z. He, L. Wu, X. Zhang, G. Li, and J. Zhou. 2011. Functional gene diversity of soil microbial communities from five oil-contaminated fields in china. ISME Journal 5 (3): 403-13.
Liu, Ruyin, Yu Zhang, Ran Ding, Dong Li, Yingxin Gao, and Min Yang. 2009. Comparison of archaeal and bacterial community structures in heavily oil-contaminated and pristine soils. Journal of Bioscience and Bioengineering 108 (5) (11): 400-7.
Mulkinsphillips, G., and J. Stewart. 1974. Effect of environmental parameters on bacterial-degradation of bunker-C oil, and hydrocarbons. Applied Microbiology 28 (6): 915-22.
Patin, Stanislav. 2004. Crude oil spills, environmental impact of. In Encyclopedia of energy., ed. Cutler J. Cleveland, 737-748. New York: Elsevier.
PRINCE, R. 1993. Petroleum spill bioremediation in marine environments. Critical Reviews in Microbiology 19 (4): 217-42.
Sadaba, R., and B. Sarinas. 2010. Fungal communities in bunker C oil-impacted sites off southern guimaras, philippines: A post-spill assessment of solar 1 oil spill. Vol. 53. Berlin,: de Gruyter.
Shin, W., and J. Pardue. 2001. Oxygen dynamics in crude oil contaminated salt marshes: I. aerobic respiration model. Environmental Technology 22 (7): 845-54.
Teralmoto, M., M. Suzuki, F. Okazaki, A. Hatmanti, and S. Harayama. 2009. Oceanobacter-related bacteria are important for the degradation of petroleum aliphatic hydrocarbons in the tropical marine environment. Microbiology 155 (10): 3362-70.
Tyagi, M., M. da Fonseca, and C. de Carvalho. 2011. Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 22 (2): 231-41.
Vila, J., J. Nieto, J. Mertens, D. Springael, and M. Grifoll. 2010. Microbial community structure of a heavy fuel oil-degrading marine consortium: Linking microbial dynamics with polycyclic aromatic hydrocarbon utilization. FEMS Microbiology Ecology 73 (2): no-362.
Xu, R., and J. Obbard. 2004. Biodegradation of polycyclic aromatic hydrocarbons in oil-contaminated beach sediments treated with nutrient amendments. Journal of Environmental Quality 33 (3): 861-7.
Edited by Yan Zhou, a student of Angela Kent at the University of Illinois at Urbana-Champaign.