Dental Water Line: Difference between revisions
Slonczewski (talk | contribs) |
|||
| (172 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Curated}} | ||
==Introduction== | |||
[[Image:Biofilm1.png|150 px|left|thumb|Biofilm Growing in Dental Waterline - magnified 60X [http://www.aquatechnology.net/Electrolyzis&dentistry.html<1>]]] | |||
Modern dentists utilize various apparatuses in treating patients in their offices. These apparatuses include but are not limited to: dental chair units, triple syringe system, high speed handpieces, ultra sonic scalers and etc [6]. These dental units provide suitable living conditions for several forms of organisms including microbes and fungi [5]. One common condition that these dental units possess is that they are always in contact with water to provide a suitable condition for the organisms. In order for these dental instruments to work properly, water-linings are installed throughout the clinic from the city-water output to the primary filtering system to each dental chair unit, which has the secondary filtering system [2]. These units, however are left unused and their powers are off during the night, which aggravates the condition of the dental water line units or facilitates the growth of bacteria in these parts [9]. Such conditions lead to biofilm formation, which is a community of microcolonies developed on the surfaces. Contamination in dental water line is mostly due to biofilm formation [18]. | |||
==Description== | |||
== | ===Physical Conditions=== | ||
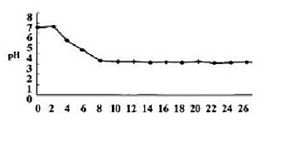
[[Image:image source.jpg|300px|thumb|pH of water discharged from the handpiece after inflow of acidic electrolyzed water [http://www.nih.go.jp/JJID/57/52.html<2>]]] | |||
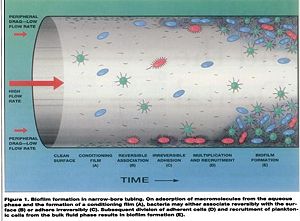
[[Image:surface area.jpg|300px|thumb|Biofilm formation in narrow-bore tubing. [17] | |||
[http://jada.ada.org/cgi/content/abstract/127/2/181 1. Copyright © 1996 American Dental Association. All rights reserved. Reproduced by permission.<3>]]] | |||
<u>'''Temperature:'''</u> | |||
Temperatures vary from one dental unit to another in most cases and also from one dental office to another. However, according to most of the references, it indicates that the temperature of this niche fall under the range of 23 to 37 degrees Celcius [4]. Some dental units have water heaters to heat the water so it would provide patients with more comfortable conditions [8]. | |||
<u>'''Moisture:'''</u> | |||
There must be a constant flow of water since most dental unit waterlines require water. | |||
''' | |||
''' | <u>'''pH:'''</u> | ||
There | The pH of this niche is neutral. There have been studies where researchers used acidic electrolyzed in dental fields to effectively disinfect the contamination at the microbial level of dental waterlines, and these studies showed that the water before it was washed with acidic electrolyzed solution had a pH of 7 [1]. | ||
''' | <u>'''Water Flow:'''</u> | ||
General water flow is more laminar (60~100mL/min) in dental unit waterlines compared to a 1/2”(25mm) diameter pipe, which has a flow rate of 5000mL/min. Dental unit waterlines have a long period of stagnation during night periods and weekends when these units are not used due to the laminar flow, therefore favoring the colonization of microbes [17]. | |||
''' | <u>'''Biofilm Surface Attachment:'''</u> | ||
As the water flows from main-fed or tank to the dental water line tubing, there is an increase in tubing surface area to water volume ratio. These environments encourage bioflim formation on the walls of tubing [18]. | |||
===Influence by Adjacent Communities=== | ===Influence by Adjacent Communities=== | ||
One of the many possibilities is contamination or microbes transferred from patient's mouth to dental apparatuses, following back siphonage, then to dental water line. Also, microbes can be transferred from the hands of dental staff while treating patients [6]. | |||
contamination or microbes | |||
===Conditions | ===Conditions Under Which the Environment Changes=== | ||
The physical conditions vary from one dental office to another, but for one particular dental office's waterline, their chemicals, water supply, nutrients, and organisms tend to stay constant most of the time. | The physical conditions vary from one dental office to another, but for one particular dental office's waterline, their chemicals, water supply, nutrients, and organisms tend to stay constant most of the time. | ||
'''Chemicals and disinfectants:''' | <u>'''Chemicals and disinfectants:'''</u> | ||
However, by using disinfectants, organisms that live in this niche may be effected (killed, numbers reduced, etc) | However, by using disinfectants, organisms that live in this niche may be effected (killed, numbers reduced, etc) [3]. | ||
For example, when the dental unit waterline is treated with chemicals or disinfectants, it is proven that the chemicals significantly alter the conditions of the waterline and reduce the number of contamination in the waterline | For example, when the dental unit waterline is treated with chemicals or disinfectants, it is proven that the chemicals significantly alter the conditions of the waterline and reduce the number of contamination in the waterline [3]. For example, according to a research, using Alpron and Bio 2000 according to the manufacture’s instructions each clinical day reduced the infection level to zero CFU/mL after two weeks [3]. Another instance of altering the condition is to use electrolyzed acidic water to disinfect the contamination, which also resulted in reduced contamination of the water line [1]. | ||
<u>'''Flushing:'''</u> | |||
It decreases the number of bacteria in the water phase. However, this reduction will be transient as the microorganisms will multiply back to high numbers quickly [9]. | |||
''' | <u>'''Filtration:'''</u> | ||
Different types of filters remove the chemicals and organic contaminants in the dental water line to reduce the formation of biofilms and inorganic deposits. [8] For example, kinetic degradation fluxion filters remove dissolved metals such as irons. This improves the quality of water in the dental unit by decreasing the contamination. [8] In addition, filtration would decrease the number of chemical treatment needed for removal of biofilm [9]. | |||
''' | <u>'''Types of Water:'''</u> | ||
The physical conditions change depend on the source of water used in the dental water line. Generally, there are four different sources of water, hard, soft, deionized, and distilled water. The study has shown that there are more bacteria recovered in the distilled water than from other three types of water. Similarly, the supply type of water can provide different conditions, which are tank, bottle, and main-fed. There are more bacteria recovered in main-fed units than those supplied in bottle or tank. However, the number of bacteria found between different source and supply of water are not significantly different [6]. | |||
==Who lives in Dental Water Line?== | ==Who lives in Dental Water Line?== | ||
===Bacteria=== | ===Bacteria=== | ||
''Achromobacter xylosoxidans'', ''Acidovorax defluvii'', ''Acidovorax'' spp., ''Acinectobacter'' spp., ''Actinomyces'' spp., ''[[Aeromonas]] hydrophila'', ''Alcaligenes dentifricans'', ''[[Bacillus]]'' spp., ''[[Bacteroides]]'' spp., ''[[Caulobacter]]'' spp., ''[[Flavobacterium]]'' spp., ''[[Fusobacterium]]'' spp., ''[[Klebsiella pneumoniae]]'', ''[[Lactobacillus]]'' spp., ''[[Legionella pneumophila]]'', ''[[Legionella]]'' spp., ''Methylophilus'' spp., ''[[Micrococcus]]'' spp., ''Moraxella'' spp., ''[[Mycobacterium]] avium'', ''Nocardia'' spp., ''[[Pasteurella]]'' spp., ''[[Porphyromonas]] gingivalis'', ''Proteus vulgaris'', ''[[Pseudomonas aeruginosa]]'', ''[[Burkholderia cepacia]]'', ''Sphigomonas paucimobilus'', ''[[Sphingomonas]]'' spp., ''[[Streptococcus]]'' spp., ''[[Staphylococcus]] aureus'', and ''[[Xanthomonas]]'' spp.[7]. | |||
''Achromobacter xylosoxidans'', ''Acidovorax defluvii'', ''Acidovorax'' spp., ''Acinectobacter'' spp., ''Actinomyces'' spp., ''[[Aeromonas]] hydrophila'', ''Alcaligenes dentifricans'', ''[[Bacillus]]'' spp., ''[[Bacteroides]]'' spp., ''[[Caulobacter]]'' spp., ''[[Flavobacterium]]'' spp., ''[[Fusobacterium]]'' spp., ''[[Klebsiella pneumoniae]]'', ''[[Lactobacillus]]'' spp., ''[[Legionella pneumophila]]'', ''[[Legionella]]'' spp., ''Methylophilus'' spp., ''[[Micrococcus]]'' spp., ''Moraxella'' spp., ''[[Mycobacterium]] avium'', ''Nocardia'' spp., ''[[Pasteurella]]'' spp., ''[[Porphyromonas]] gingivalis'', ''Proteus vulgaris'', ''[[Pseudomonas aeruginosa]]'', ''[[Burkholderia cepacia]]'', ''Sphigomonas paucimobilus'', ''[[Sphingomonas]]'' spp., ''[[Streptococcus]]'' spp., ''[[Staphylococcus]] aureus'', and ''[[Xanthomonas]]'' spp. [7]. | |||
Among bacteria that are listed above, there are four most commonly discussed bacteria within biofilms of Dental Water Line. They are ''[[Legionella pneumophila]]'', [[Mycobacterium|''Mycobacterium'' spp.]], [[Pseudomonas aeruginosa]], and [[Staphylococcus|''Staphylococcus'' spp.]]. | Among bacteria that are listed above, there are four most commonly discussed bacteria within biofilms of Dental Water Line. They are ''[[Legionella pneumophila]]'', [[Mycobacterium|''Mycobacterium'' spp.]], [[Pseudomonas aeruginosa]], and [[Staphylococcus|''Staphylococcus'' spp.]]. | ||
[[Image: | {| border="2" cellpadding="3" | ||
| align="center" style="background:#f0f0f0;"|'''Microbe''' | |||
| align="center" style="background:#f0f0f0;"|'''Figure''' | |||
| align="center" style="background:#f0f0f0;"|'''Description''' | |||
|- | |||
| '''''[[Legionella pneumophila]]'''''|| | |||
[[Image:Legionella_pneumophila.jpg|thumb|200px|right|''Legionella pneumophila''. [http://education.denniskunkel.com/catalog/product_info.php?products_id=9154 Dennis Kunkel Microscopy, Inc.<4>]]] | |||
||L. pneumophila is a gram-negative bacterium which spreads via the air-conditioning system. They are called chemo-organotrophs because they are capable of using certain amino acids as primary carbon and energy sources. Their cell walls are unique in a way that they contain a huge amount of branched fatty acids. Once they enter the cells, they reside and multiply in numbers within a membrane-bound compartment. A respiratory trace and the lungs are the main sites of infections. Legionnaire’s disease is an atypical lung infection caused by Legionella pneumophila [19]. | |||
|- | |||
| [[Mycobacterium|'''''Mycobacterium'' spp.''']]|| | |||
[[Image:Mycobacterium_avium.jpg|thumb|200px|right|''Mycobacterium avium''. [http://education.denniskunkel.com/catalog/product_info.php?products_id=508 Dennis Kunkel Microscopy, Inc.<5>]]] | |||
||It is a bacterial genus which contains a vast number of species. The best known species are M. leprae and M. Tuberculosis which cause leprosy and tuberculosis respectively. In their cell wall, it consists of special fatty molecules called mycolic acids, and these complexes make the cell walls less permeable. They are aerobic, non-motile rods [20]. | |||
|- | |||
| '''''[[Pseudomonas aeruginosa]]'''''|| | |||
[[Image:Pseudomonas_aeruginosa.jpg|thumb|200px|right|''Pseudomonas aeruginosa''. [http://education.denniskunkel.com/catalog/product_info.php?products_id=10044 Dennis Kunkel Microscopy, Inc.<6>]]] | |||
||It is a heterogeneous genus comprising gram-negative, aerobic, rod-shaped bacteria. They exhibit motility since they have one or more polar flagella. They also contain fimbriae as an attachment to surfaces of other organisms. They grow fastest at the room temperature [21]. Pseudomonas aeruginosa is the best known and the most virulent pathogenic pseudomonad. It is known as one of the most abundant life forms on the planet. Despite their widespread distribution, P. Aeruginosa infections are relatively rare [22]. | |||
|- | |||
| [[Staphylococcus|'''''Staphylococcus'' spp.''']]|| | |||
[[Image:Staphylococcus_aureus.jpg|thumb|200px|right|''Staphylococcus aureus''. [http://education.denniskunkel.com/catalog/product_info.php?products_id=1404 Dennis Kunkel Microscopy, Inc.<7>]]] | |||
||They are small cocci that exist in irregular clumps or grape-like clusters. There are three main species of Staphylococcus that share morphological and biochemical features. The pathogenic organisms produce many extracellular products known to play as a factor that leaves effects on humans [23]. | |||
|} | |||
===Fungi and Protozoa=== | |||
<u>'''Fungi:'''</u> | |||
''Phoma spp., [[Penicillium]] spp., [[Cladosporium]] spp., Alternaris spp., and Scopulariopsis spp.'' [7]. | |||
<u>'''Protozoa:'''</u> | |||
''Acanthamoeba spp., [[Cryptosporidium]] spp., Microsporidium spp., and [[Giardia]] spp.'' [7]. | |||
===Interaction Between Each Other=== | |||
Biofilm provides a suitable niche for conjugation to occur. Bacteria cells formed in biofilms will transfer genetic elements at a greater rate than those in the planktonic phase. This is primarily due to the close cell to cell contact and the minimal shear [9]. | |||
With this closely packed community, there are several possible ways that bacteria inhibits the forming of biofilms. ''[[Pseudomonas aeruginosa]]'' exhibit the cell-cell signaling, which plays a major role in attachment and detachment. At higher densities of biofilm colonies, the signal molecules, such as homoserine lacones in Gram negative bacteria and peptides in Gram positive bacteria, reach sufficient concentrations to activate genes involved in biofilm break-down. Similarly, ''[[Streptococcus]]'' cristatus is able to inhibit attachment of ''[[Porphyromonas]] gingivalis'' to a plaque biofilm. ''[[Aeromonas]] hydrophila'' is known to form biofilms in water systems. However, mutant forms of ''A. hydrophila'' were weakened in cell-cell communication, which resulted in their incapability to form biofilms [9]. | |||
As diverse microorganisms engage in forming the biofilm, the interactions may include incidents of antagonism and commensalism. In case of antagonism, there may be competition and predation due to bacteriovores, like free-living protozoa, which have been known to be 300 times more in number in Dental Unit Water than in city water, and bacteriophage that attacks certain bacteria in the biofilm [11]. | |||
[ | There are also some commensalism cases in that several free-living amoebae, which have been found that while, encysted, they protect the inner microorganism from disinfectants and other boicides, and the microorganism may include legionella and other pathogenic microbes [12]. | ||
There have not been many studies done onto how a certain bacterium is interacting with other microbial cells specifically with the dental unit water line. | |||
'' | ''[[Pseudomonas fluorescens]]'' and ''[[Bacillus cereus]]'' show significant differences in behavior and composition of planktonic and sessil dual species communities of them. Iron availability was used to measure differences in planktonic mixed growth of ''P. fluorescens'' and ''B. cereus''. Under iron deficient condition, ''P. fluorescens'' prevented the vegetative growth of ''B. cereus''. | ||
Dual biofilms were formed in a greater extent by ''B. cereus''. B.cereus, being the primary surface colonizer, attached more effectively than ''P. fluorescens''. Thus, the biofilms had a structure with a mid-layer composed of ''P. fluorescens'', which was surrounded by two layers of ''B. cereus''. The dominance of ''B. cereus'' in the outer layer of the dual biofilm is due to the constant supply of medium, and such flow may minimize the inhibitory factors formed by ''P. fluorescens''. Also, the two specie dual biofilms had shown its weakened physical stability compared to that of the single species biofilms[13]. In other words, there is some antagonism relationship, or some similar effect between ''P. fluorescens'' and ''B. cereus'' in biofilms. | |||
===Do the microbes change their environment?=== | |||
<u>'''Binding to surface/Biofilm formation:'''</u> | |||
Hypothetically, the flow rate situated next to the substratum-liquid interface is insignificant. This area is coined with the term “hydrodynamic boundary layer” whose dimensions are inversely related to the water speed. Once a microbial cell has arrived at the hydrodynamic boundary layer, it can start the process of attacment [9]. | |||
The initial attachment of bacteria to a surface is often descrbied by the DLVO Theory proposed by Derjaquin and Landau and Verwey and Overbeek. Once a cell is inside the hydrodynamic boundary layer, it experiences an area of non-specific attraction about 10~20nm from the surface. This area is created due to the Van der Wall’s forces of attraction and electrostatic repulsion. At this stage, various properties of bacterial cell affects adhesion: Hydrophobic non-flagella appendages such as fimbrae help in cell attachment by overcoming the electrostatic repulsion barrier that is located between the cell and the substratum; Lipopolysaccharides and exo-polysaccharides, protein, and protein-carbohydrate interactions also play important roles in the process of cell attachment at this stage [9]. | |||
Overall microbial attachment involves a dynamic complex process. This can be affected by different variables such as flow rate, surface roughness and hydrophobicity, and the presence and properties of conditioning films. DUWS contains several features that encourage increase in bacterial contamination. Since the tubing of DUWS is composed of polyurethane and polyvinylchloride, it can serve as nutrient sources for the bacteria. Moreover, DUWS is faced with long periods of stagnation that will encourage biofilm formation as well [9]. | |||
When the cell is attched to a surface, cells start to grow quickly and form microcolony. Then secondary colonies, which are colonies that lack the ability to initially attach to a surface by themselvch to the primary colonisers [9]. | |||
A biofilm community can provide an ideal condition for the exchange of extrachromosomal DNA and plasmids. For example, plasmid recipient strains have been proven to demonstrate increased biofilm growth (penicillin binding proteins and IgA proteases) [9]. | |||
Lastly, when the biofilm is at the climax number of community, individual cells and parts of the biofilm can be lost from the surface due to the loss of nutrient, quorum sensing or shear forces. This occurs when a newly divided daughter cell is shed from the outermost layer of the biofilm. Detachment from physical process can result from erision or shear, rapid loss and abrasion. Even though high shear forces may reduce the biofilm, the laminar flow rates in DUWS are insufficient to result in a great loss of the biofilm structure (DUWS has the maximum flow rate of 100mL/min) [9]. | |||
==Prevention and Solutions for Contaminated Dental Water Line== | |||
[[Image:Sabri.jpg|400 px|thumb|Epifluorescence microscopy of embedded biofilm in the different tubing samples (AWT = Air Water Tubing, PT = Patient Tubing, MWP = Main Water Pipe)[http://www.springerlink.com/content/f40162v538247542/<8>]]] | |||
===Non-chemical Treatment=== | |||
<u>'''DUWS engineering and redesigning:'''</u> | |||
DUWS manufacturing is critical to achieve the improvements of water quality. Designing and evaluating DUWS is required to solve the problem of biofouling in the pipes. The length of the stagnant section can be reduced by redesigning the units and keep the water flow continuous. Also, the materials used could be re-evaluated to provide tubing that has less susceptibility to biofouling [10]. | |||
<u>'''Flushing:'''</u> | |||
Flushing the water line for several minutes before the first patient and for 20-30s between patients is recommended so that the number of bacteria in the water line can be decreased. However, this reduction is only temporary because the microorganisms will multiply back in a very short time. Therefore, an alternative strategy could be a flushing the dental unit at the end of the day and dry it overnight to reduce development of the biofilm [10]. | |||
<u>'''Filtration:'''</u> | |||
In-line 0.2 ㎛ membrane filters is used for the filtration of the dental unit water when they need to reduce the need for chemical treatment. The length of the DUWS does not matter too much, but the system beyond the filter will be susceptible to microbial contamination [10]. | |||
<u>'''Ultraviolet Light (UV):'''</u> | |||
As the water flows out over the UV lamp, the bacterial numbers will be reduced. It is mainly supplemented by filtration and/or alternating chemical treatment [10]. | |||
<u>'''Physical Cleaning:'''</u> | |||
This method uses sponges or balls by making them pass through the pipeline at high pressure in order to remove the biofilm and destroy biofouling. (This technique is not applicable to a current design of DUWS) [10]. | |||
===Chemical Treatment=== | |||
Many chemicals treatments are applied to treat the dental unit water. The products are used in the equipment to remove and kill biofilms. The common products consist of chemical compounds such as sodium hypochlorite, chlorine dioxide, peroxides, and citric acid. Other chemicals include electrochemically activated water for microbial control. Also, the use of biocides (a poisonous substance to living organisms) is one of the several proposed methods to reduce the contamination in the dental water line, such as removal of bioflim. When the NaOCl plus Phe combined chemical is added to the dental water lines, there has been an 85% to 95% decrease in bioflim formation and significant increase in bioflim removal from the surface [10]. | |||
Figure 9 above shows the epifuluoroscence microscopy of the biofilm in three different tubings, which are Air and Water Tubing (AWT), Patient Tubing (PT), and Main Water Pipe (MWP). Column (A) shows the thick colonization of biofilm without any treatment, column (B) shows a partially reduced biofilm as all biocides have been flushed through tubing samples, and column (C) shows nearly no biofilm after flushing with only NaOCl with Phe [10]. | |||
==Current Research== | |||
'''<u>First Topic: Effects of Various disinfectants.</u>''' | |||
''' Article: M. Özcan, Y. Kulak and E. Kazazoglu "The effect of disinfectant agents in eliminating the contamination of dental unit water." <u>Journal of Oral Rehabilitation</u>, 30; p.290–294.''' | |||
Most serious spread of these microbial organisms arise from forming a community, or biofilm, which helps the organisms to survive washing or even drying up in these dental units. Since this can be detrimental to patients,dental offices have been trying to lower or even to get rid of these microbial organisms living in the dental unit waterline niche. Various methods including using electrolyzed acidic water and various chemical products are used to treat these waterlines, and this research paper focuses on two disinfectants (Bio® 2000 and Alpron®) and their effects on these waterlines. The unit for testing these waterlines’ condition was CFU (colony forming units) per mL. In order to effectively carry out the test, these two disinfectants were used according to the manufacturer’s instructions. The waterlines were treated on a daily basis at the end of each day by using the two disinfectants in the waterlines. Baseline, daily samples of 100 mL for the first week and the second samples for the week thereafter were plated on different agar plates. This resulted in: for Bio 2000, baseline had the mean value of CFU/mL of 36.25, first week and second week had 0; for Alpron, baseline had the mean value of CFU/mL of 760, first week had 0.75, and second week had 0. According to these results, the baseline had higher concentration of infection than the first week and second week of treatment for both Bio 2000 and Alpron. Moreover, since these two disinfectants were proved to be effective when used for more than two weeks, dental offices should be encouraged to use disinfectants to reduce the CFU/mL and detrimental health hazards [3]. | |||
'''<u>Second Topic: Presence of ''Pseudomonas aeruginosa'' in the dental unit waterline systems.</u>''' | '''<u>Second Topic: Presence of ''Pseudomonas aeruginosa'' in the dental unit waterline systems.</u>''' | ||
'''Article: Al-Hiyasat AS, ''et al''. "The presence of ''Pseudomonas aeruginosa'' in the dental unit waterline systems of teaching clincs." <u>International Journal of Dental Hygiene</u> 5 (2007): p.36-44. | |||
It has been known from several former studies that there are pathogenic and opportunistic bacteria, such as Pseudomonas aeruginosa, Legionella pneumophila, and Staphylococcus aureus in DUW. This study was to examine the Pseudomonas aeruginosa contamination of DUW (Dental Unit water) at a Dental Teaching Center in Jordan. And to evaluate the extent of P. aeruginosa contamination, which is widely exposed to dental staff. Total of 30 dental units were used to collect water samples from three teaching clinics, conservative dentistry, periodontology, and prosthodontics. Samples were collected at the beginning of the working day, after 2 minutes of flushing, and at the midday from the air/water syringe, high-speed handpiece and water cup filler. Pseudomonas aeruginosa isolated from the dental unit water has shown to cause infection. Two patients’ mouth infection had same strain that has been isolated from the dental unit water system. The results show that at the beginning of the working day, 86.7% of the dental units examined were contaminated with P. aeruginosa. And flushing the dental water line for 2 minutes significantly decreased the amount of P. aeruginosa. Another information attained from the study was that the source of contamination in related to P. aeruginosa could be from patients’ mouths, meaning that the anti-retraction valves in dental units have failed to prevent the back flow of bacteria [14]. | |||
'''<u>Third Topic: Legionella Contamination in Dental unit water.</u>''' | |||
'''Article: Zanetti F, ''et al''."Water characteristics associated with the occurrence of Legionella Pneumophila in dental units." <u>European Journal of Oral Sciences</u>, 108; p.22-28''' | |||
Legionella species are pathogenic micro-organisms which may be present in a variety of water systems. Majority of the Legionella are considered to be biofilm associated because they are more easily found in swab samples of biofilm than from flowing water. 101 water samples were taken from the incoming tap water, oral rinsing cup, air-water syringe, ultrasonic scaler, Legionella pneumophila was found in 22 samples. There was a greater frequency and concentration of L. Pnuemophila in the incoming water compared to the out-flowing water in the dental units. It was because of the complex structure of dental chair equipment. It helps the water to stagnate in the waterlines, leading to the formation of a biofilm. Through this study, it was also found that the organic matter enhances the occurrence of Legionella and the pH does not present a critical factor that regulates the growth of Legionella pneumophila. In addition, bacteria such as Psudomonas spp and Aeromonas spp have been tested to be capable of inhibiting Legionella. | Legionella species are pathogenic micro-organisms which may be present in a variety of water systems. Majority of the Legionella are considered to be biofilm associated because they are more easily found in swab samples of biofilm than from flowing water. 101 water samples were taken from the incoming tap water, oral rinsing cup, air-water syringe, ultrasonic scaler, Legionella pneumophila was found in 22 samples. There was a greater frequency and concentration of L. Pnuemophila in the incoming water compared to the out-flowing water in the dental units. It was because of the complex structure of dental chair equipment. It helps the water to stagnate in the waterlines, leading to the formation of a biofilm. Through this study, it was also found that the organic matter enhances the occurrence of Legionella and the pH does not present a critical factor that regulates the growth of Legionella pneumophila. In addition, bacteria such as Psudomonas spp and Aeromonas spp have been tested to be capable of inhibiting Legionella. | ||
The primary route of infection of L. Pneumophila is via inhalation. Therefore, the presence of L. pneumophila in water used for dental treatments may threaten both patients and the dental team. In order to solve this problem, a disinfectant must be used to reduce the number of biofilm formation and also tubing system of the dental units need be tested against it | The primary route of infection of L. Pneumophila is via inhalation. Therefore, the presence of L. pneumophila in water used for dental treatments may threaten both patients and the dental team. In order to solve this problem, a disinfectant must be used to reduce the number of biofilm formation and also tubing system of the dental units need be tested against it [15]. | ||
'''<u>Fourth Topic: Engineering of Dental Chair Unit.</u>''' | |||
''' | ''' Article: D.C. Coleman, ''et al''."The Role of Manufacturers in Reducing biofilms in Dental Chair Waterlines." <u>Journal of Dentistry</u>, 35 (2007): p.701-711.''' | ||
There is continuing research on how dental chair unit manufacturer would help to resolve the problem of biofilm formation in dental unit water. One of the improvements in manufacturing the dental chair unit to decrease the formation of bioflim in the dental unit water is, fully automated dental unit water disinfection systems that are developed for long-term effects. In prevention of back-siphonage of oral fluids into the water, improved anti-retraction devices are installed in the dental chair unit. Also, an air gap is installed to separate the dental unit waters in dental chair units from municipal mains water supplies. Researchers also found that the quality of water supply can be controlled in pre-treatment by using water softener and filtration, which removes the organic and inorganic materials in the dental unit water. They discovered that many of the large dental clinics are equipped with these systems in the dental chair unit. However, it is most important to perform periodic testing and maintenance to prevent bioflim formation [8]. | |||
'''<u>Fifth Topic: Unusual Fungus in Dental Water Line.</u>''' | |||
''' | '''Article: Porteous, Nuala B., ''et al''. “Isolation of an Unusual Fungus in Treated Dental Unit Waterlines.” <u>Journal of the American Dental Association</u> 134. 7 (2003): p.853-858.''' | ||
' | [[Image:Porteous.jpg|800px|left|thumb|[16], [http://jada.ada.org/cgi/content/full/134/7/853/ Copyright © 2003 American Dental Association. All rights reserved. Reproduced by permission. <9>]]] | ||
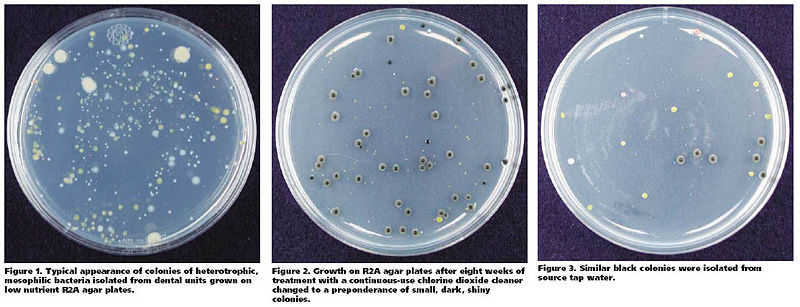
Many microbes reside in dental water line. Although most of such microbes are known to be non-pathogenic, heterotrophic bacteria, some of them like ''Legionella'' and ''Pseudonomas'' are opportunistic pathogens that are able to cause respiratory diseases such as pneumonia. The American Dental Association(ADA) recommends keeping colony-forming unit(CFU) level of non-surgical dental water lower than 200 CFU/ml.To examine the antibacterial effect of a continuous-use, stabilized chlorine dioxide compound, Porteous ''et al''. organized a study where they used six dental units, all furnished with self-contained water system. Three of them were filled with a 1:10 dilution of continuous-use, stabilized chlorine dioxide compound, and the other three were used as control group. The authors sampled water from each of dental units weekly. The samples were properly cultured on R2A Agar for a week.Until the eighth week, bacterial colonies were isolated and observed from both treatment and control groups. In the ninth week, however, small, dark colonies which seemed to be fungal started to appear in a sample from one of the treated units. Porteous ''et al''. also isolated colonies resembling such appearance from elsewhere in the same dental unit. Such fungus-like colonies were found in all the other treated units. After DNA sequencing process, the organism is diagnosed to be ''Exophiala mesophila''. No fungus was isolated from any of dental units in control group which proposes that continuous use of stabilized chlorine dioxide compound may result in multiplication of a fungus in dental water line. [16] | |||
==Summary about the Dental Water Line niche== | ==Summary about the Dental Water Line niche== | ||
Dental chair units provide suitable environment for microbes to grow and to form biofilms. Biofilm formation in dental unit water has been understood by several theories, such as DLVO, and it has been researched through numerous experiments. According to the researches, there are interactions among the micro-organisms within biofilms and they may experience commensalism or antagonism. Those biofilms help microbes to survive well in the dental unit systems. The physical and chemical conditions of the dental unit waterlines differ from one to another, however, they maintain their stability most of the time. There are many bacteria, several fungi, and protozoa are found in the DUWs. Most common bacteria in dental waterlines are ''[[Mycobacterium]]'' spp., ''Legionelle'' spp., ''[[Pseudomonas]]'' spp., and ''[[Staphylococcus]]'' spp. Many studies have introduced effective methods to decontaminate the dental unit waterlines, and they can be divided into chemical and non-chemical treatment. Even though there have been many studies done in this field, there are still researches going on to improve the environment of DUWs. | |||
==References== | ==References== | ||
(1) Kohno, S., Kawata T., ''et al''. "Bactericidal Effects of Acidic Electrolyzed Water on the Dental Unit Waterline." <U>Shinya Jpn J Infect Dis.</U> Department of Orthodontics and Craniofacial Developmental Biology, Hiroshima University Graduate School of Biomedical Sciences. 57 (2004):52-54.29 Aug. 2008. <http://www.nih.go.jp/JJID/57/52.html> | |||
(2) Kim, Eugene. D.D.S. Personal interview. 11 August 2008. | |||
(3) Ozcan, M., Y. Kulak, and V. Kazazoglu. "The effect of disinfectant agents in eliminating the contamination of dental unit water." <u>Journal of Oral Rehabilitation</u> John Wiley & Sons, Inc. 30 (2003): 290–294. 29 Aug. 2008 <http://www3.interscience.wiley.com/cgi-bin/fulltext/118839807/HTMLSTART> | |||
(4) Robert M, J. Barbeau, A. Prévost, and R. Charland. "Dental unit water lines: A propitious environment for bacterial colonization." <u>Legionnaries' Desease.</u> 29 Aug. 2008 | |||
(5) Souza-Gugelmin, Maria Cristina Monteiro, ''et al''. "Microbial contamination in dental unit waterlines." <u>Braz. Dent. Journal.</u> Scielo.Br. 14, (2003). 29 Aug. 2008 <http://www.scielo.br/scielo.php?pid=S0103-64402003000100010&script=sci_arttext&tlng=en> | |||
(6) Walker, James T., David J. Bradshaw, Allan M. Bennett, Martin R. Fulford, Michael V. Martin, and Philip D. Marsh. "Microbial Biofilm Formation and Contamination of Dental-Unit Water Systems in General Dental Practice." <u>Appl Environ Microbiol</u> 2000 August; 66(8): 3363-3367 <http://aem.asm.org/cgi/content/full/66/8/3363?view=long&pmid=10919792> | |||
(7) Pankhurst, Caroline L., and N.W. Johnson. "Microbial contamination of dental unit waterlines: the scientific argument." <u>International Dental Journal</u> 48 (1998): 359–368. 28 August 2008 <http://www.ncbi.nlm.nih.gov/pubmed/9779119?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum> | |||
(8) Coleman, D.C., M.J. O'Donnell, A.C. Shore, J. Swan, R.J. Russell. "The Role of Manufacturers in Reducing Bioflims in Dental Chair Waterlines." <u>Journal of Dentistry</u> 35 (2007) 701-711. <http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T86-4P0N91R-1&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000059602&_version=1&_urlVersion=0&_userid=4429&md5=69a82c61c2a64af5623cc97591fb522f > | |||
(9) Walker, J.T., and P.D. Marsh. "A review of biofilms and their role in microbial contamination of dental unit water systems(DUWS)." <u>International Biodeterioration & Biodegradation</u> 54 (2004): 87 – 98. 28 August 2008 <http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VG6-4CC2YY4-1&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=4429&md5=158eca25a4e362f1e21594714380c638> | |||
(10) Liaqat, I., and A.N. Sabri. "Effect of Biocides on Biofilm Bacteria from Dental Unit Water Lines." <u>Current Microbiology</u> 56 (2008): 619–624. 28 August 2008 <http://www.ncbi.nlm.nih.gov/pubmed/18322732> | |||
(11) Walker, J.T., and P.D. Marsh. "Microbial biofilm formation in DUWS and their control using disinfectants." <u>Journal of Dentistry</u> 35 (2007): 721–730. 28 August 2008 <http://linkinghub.elsevier.com/retrieve/pii/S0300-5712(07)00132-7> | |||
( | (12) Singh, T., and Coogan, M.M. "Isolation of pathogenic Legionella species and legionella-laden amoebae in dental unit waterlines" <u>Journal of Hospital Infection</u> 61 (2005): 257–262. 28 Aug. 2008 | ||
( | <http://linkinghub.elsevier.com/retrieve/pii/S0195-6701(05)00191-X> | ||
(13) Simoes M., ''et al''. "Antagonism between ''Bacillus cereus'' and ''Pseudomonas fluorescens'' in planktonic systems and in biofilms"<u>Biofouling</u> 24 (2008):5,339 — 349. 28 Aug. 2008 <http://www.informaworld.com/smpp/content~db=all?content=10.1080/08927010802239154> | |||
(14) Al-Hiyasat AS, ''et al''. "The presence of ''Pseudomonas aeruginosa'' in the dental unit waterline systems of teaching clincs." <u>International Journal of Dental Hygiene</u> 5 (2007): p.36-44. <http://www3.interscience.wiley.com/journal/118537317/abstract> | |||
(15) Zanetti F, Stampi S, De Luca G, et al., "Water Characteristics associated with the occurrence of Legionella Pneumophila in dental units." <u>European Journal of Oral Sciences</u>. 108; p.22-28 <http://www.ncbi.nlm.nih.gov/pubmed/10706473> | |||
(16) Porteous, Nuala B., ''et al''. “Isolation of an Unusual Fungus in Treated Dental Unit Waterlines.” <u>Journal of the American Dental Association</u> 134. 7 (2003): 853-858. 29 August 2008 <http://jada.ada.org/cgi/content/full/134/7/853>. | |||
( | (17) Shearer, BG. "Biofilm and the dental office." <u>Journal of the American Dental Association</u> 127. 2 (1996): 181-189. 29 August 2008 <http://jada.ada.org/cgi/reprint/127/2/181> | ||
(18) Franco, F.F.S., D. Spratt, J.C. Leao and S.R. Porter. "Biofilm Formation and Control in Dental Unit Waterlines." <u>Bioflims</u> 2 (2005): 9-17. | |||
( | (19) Sankaran, Neeraja. “''Legionella pneumophila''.” <u>Microbes and People: an A-Z of Microorganisms in Our Lives</u>. Phoenix: Oryx Press, 2000. 151-152. | ||
( | (20) ---. “''Mycobacterium''.” <u>Microbes and People: an A-Z of Microorganisms in Our Lives</u>. Phoenix: Oryx Press, 2000. 169. | ||
( | (21) ---. “''Pseudomonas''.” <u>Microbes and People: an A-Z of Microorganisms in Our Lives</u>. Phoenix: Oryx Press, 2000. 205-206. | ||
( | (22) ---. “''Pseudomonas aeruginosa''.” <u>Microbes and People: an A-Z of Microorganisms in Our Lives</u>. Phoenix: Oryx Press, 2000. 205-206. | ||
( | (23) ---. “''Staphylococcus''.” <u>Microbes and People: an A-Z of Microorganisms in Our Lives</u>. Phoenix: Oryx Press, 2000. 231-232. | ||
( | (24) Atlas,R. M., ''et al''. "Legionella Contamination of dental-unit waters." <u>Applied Environmental Microbiology</u> 61 (1995):1208-1213. 28 August 2008 <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=167375> | ||
''Edited by Jane Choi, Bo R. Heo, Jinsoo Lee, Soh Yun Lee, and So Young Moon, students of [mailto:ralarsen@ucsd.edu Rachel Larsen]'' | ''Edited by Jane Choi, Bo R. Heo, Jinsoo Lee, Soh Yun Lee, and So Young Moon, students of [mailto:ralarsen@ucsd.edu Rachel Larsen]'' | ||
Latest revision as of 18:05, 29 January 2012
Introduction
Modern dentists utilize various apparatuses in treating patients in their offices. These apparatuses include but are not limited to: dental chair units, triple syringe system, high speed handpieces, ultra sonic scalers and etc [6]. These dental units provide suitable living conditions for several forms of organisms including microbes and fungi [5]. One common condition that these dental units possess is that they are always in contact with water to provide a suitable condition for the organisms. In order for these dental instruments to work properly, water-linings are installed throughout the clinic from the city-water output to the primary filtering system to each dental chair unit, which has the secondary filtering system [2]. These units, however are left unused and their powers are off during the night, which aggravates the condition of the dental water line units or facilitates the growth of bacteria in these parts [9]. Such conditions lead to biofilm formation, which is a community of microcolonies developed on the surfaces. Contamination in dental water line is mostly due to biofilm formation [18].
Description
Physical Conditions
Temperature: Temperatures vary from one dental unit to another in most cases and also from one dental office to another. However, according to most of the references, it indicates that the temperature of this niche fall under the range of 23 to 37 degrees Celcius [4]. Some dental units have water heaters to heat the water so it would provide patients with more comfortable conditions [8].
Moisture: There must be a constant flow of water since most dental unit waterlines require water.
pH: The pH of this niche is neutral. There have been studies where researchers used acidic electrolyzed in dental fields to effectively disinfect the contamination at the microbial level of dental waterlines, and these studies showed that the water before it was washed with acidic electrolyzed solution had a pH of 7 [1].
Water Flow: General water flow is more laminar (60~100mL/min) in dental unit waterlines compared to a 1/2”(25mm) diameter pipe, which has a flow rate of 5000mL/min. Dental unit waterlines have a long period of stagnation during night periods and weekends when these units are not used due to the laminar flow, therefore favoring the colonization of microbes [17].
Biofilm Surface Attachment: As the water flows from main-fed or tank to the dental water line tubing, there is an increase in tubing surface area to water volume ratio. These environments encourage bioflim formation on the walls of tubing [18].
Influence by Adjacent Communities
One of the many possibilities is contamination or microbes transferred from patient's mouth to dental apparatuses, following back siphonage, then to dental water line. Also, microbes can be transferred from the hands of dental staff while treating patients [6].
Conditions Under Which the Environment Changes
The physical conditions vary from one dental office to another, but for one particular dental office's waterline, their chemicals, water supply, nutrients, and organisms tend to stay constant most of the time.
Chemicals and disinfectants: However, by using disinfectants, organisms that live in this niche may be effected (killed, numbers reduced, etc) [3]. For example, when the dental unit waterline is treated with chemicals or disinfectants, it is proven that the chemicals significantly alter the conditions of the waterline and reduce the number of contamination in the waterline [3]. For example, according to a research, using Alpron and Bio 2000 according to the manufacture’s instructions each clinical day reduced the infection level to zero CFU/mL after two weeks [3]. Another instance of altering the condition is to use electrolyzed acidic water to disinfect the contamination, which also resulted in reduced contamination of the water line [1].
Flushing: It decreases the number of bacteria in the water phase. However, this reduction will be transient as the microorganisms will multiply back to high numbers quickly [9].
Filtration: Different types of filters remove the chemicals and organic contaminants in the dental water line to reduce the formation of biofilms and inorganic deposits. [8] For example, kinetic degradation fluxion filters remove dissolved metals such as irons. This improves the quality of water in the dental unit by decreasing the contamination. [8] In addition, filtration would decrease the number of chemical treatment needed for removal of biofilm [9].
Types of Water: The physical conditions change depend on the source of water used in the dental water line. Generally, there are four different sources of water, hard, soft, deionized, and distilled water. The study has shown that there are more bacteria recovered in the distilled water than from other three types of water. Similarly, the supply type of water can provide different conditions, which are tank, bottle, and main-fed. There are more bacteria recovered in main-fed units than those supplied in bottle or tank. However, the number of bacteria found between different source and supply of water are not significantly different [6].
Who lives in Dental Water Line?
Bacteria
Achromobacter xylosoxidans, Acidovorax defluvii, Acidovorax spp., Acinectobacter spp., Actinomyces spp., Aeromonas hydrophila, Alcaligenes dentifricans, Bacillus spp., Bacteroides spp., Caulobacter spp., Flavobacterium spp., Fusobacterium spp., Klebsiella pneumoniae, Lactobacillus spp., Legionella pneumophila, Legionella spp., Methylophilus spp., Micrococcus spp., Moraxella spp., Mycobacterium avium, Nocardia spp., Pasteurella spp., Porphyromonas gingivalis, Proteus vulgaris, Pseudomonas aeruginosa, Burkholderia cepacia, Sphigomonas paucimobilus, Sphingomonas spp., Streptococcus spp., Staphylococcus aureus, and Xanthomonas spp. [7].
Among bacteria that are listed above, there are four most commonly discussed bacteria within biofilms of Dental Water Line. They are Legionella pneumophila, Mycobacterium spp., Pseudomonas aeruginosa, and Staphylococcus spp..
| Microbe | Figure | Description |
| Legionella pneumophila |
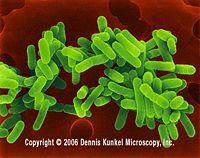 Legionella pneumophila. Dennis Kunkel Microscopy, Inc.<4> |
L. pneumophila is a gram-negative bacterium which spreads via the air-conditioning system. They are called chemo-organotrophs because they are capable of using certain amino acids as primary carbon and energy sources. Their cell walls are unique in a way that they contain a huge amount of branched fatty acids. Once they enter the cells, they reside and multiply in numbers within a membrane-bound compartment. A respiratory trace and the lungs are the main sites of infections. Legionnaire’s disease is an atypical lung infection caused by Legionella pneumophila [19]. |
| Mycobacterium spp. |
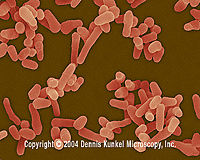 Mycobacterium avium. Dennis Kunkel Microscopy, Inc.<5> |
It is a bacterial genus which contains a vast number of species. The best known species are M. leprae and M. Tuberculosis which cause leprosy and tuberculosis respectively. In their cell wall, it consists of special fatty molecules called mycolic acids, and these complexes make the cell walls less permeable. They are aerobic, non-motile rods [20]. |
| Pseudomonas aeruginosa |
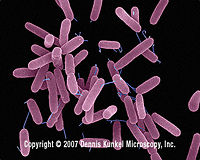 Pseudomonas aeruginosa. Dennis Kunkel Microscopy, Inc.<6> |
It is a heterogeneous genus comprising gram-negative, aerobic, rod-shaped bacteria. They exhibit motility since they have one or more polar flagella. They also contain fimbriae as an attachment to surfaces of other organisms. They grow fastest at the room temperature [21]. Pseudomonas aeruginosa is the best known and the most virulent pathogenic pseudomonad. It is known as one of the most abundant life forms on the planet. Despite their widespread distribution, P. Aeruginosa infections are relatively rare [22]. |
| Staphylococcus spp. |
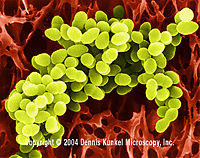 Staphylococcus aureus. Dennis Kunkel Microscopy, Inc.<7> |
They are small cocci that exist in irregular clumps or grape-like clusters. There are three main species of Staphylococcus that share morphological and biochemical features. The pathogenic organisms produce many extracellular products known to play as a factor that leaves effects on humans [23]. |
Fungi and Protozoa
Fungi: Phoma spp., Penicillium spp., Cladosporium spp., Alternaris spp., and Scopulariopsis spp. [7].
Protozoa: Acanthamoeba spp., Cryptosporidium spp., Microsporidium spp., and Giardia spp. [7].
Interaction Between Each Other
Biofilm provides a suitable niche for conjugation to occur. Bacteria cells formed in biofilms will transfer genetic elements at a greater rate than those in the planktonic phase. This is primarily due to the close cell to cell contact and the minimal shear [9].
With this closely packed community, there are several possible ways that bacteria inhibits the forming of biofilms. Pseudomonas aeruginosa exhibit the cell-cell signaling, which plays a major role in attachment and detachment. At higher densities of biofilm colonies, the signal molecules, such as homoserine lacones in Gram negative bacteria and peptides in Gram positive bacteria, reach sufficient concentrations to activate genes involved in biofilm break-down. Similarly, Streptococcus cristatus is able to inhibit attachment of Porphyromonas gingivalis to a plaque biofilm. Aeromonas hydrophila is known to form biofilms in water systems. However, mutant forms of A. hydrophila were weakened in cell-cell communication, which resulted in their incapability to form biofilms [9].
As diverse microorganisms engage in forming the biofilm, the interactions may include incidents of antagonism and commensalism. In case of antagonism, there may be competition and predation due to bacteriovores, like free-living protozoa, which have been known to be 300 times more in number in Dental Unit Water than in city water, and bacteriophage that attacks certain bacteria in the biofilm [11].
There are also some commensalism cases in that several free-living amoebae, which have been found that while, encysted, they protect the inner microorganism from disinfectants and other boicides, and the microorganism may include legionella and other pathogenic microbes [12].
There have not been many studies done onto how a certain bacterium is interacting with other microbial cells specifically with the dental unit water line. Pseudomonas fluorescens and Bacillus cereus show significant differences in behavior and composition of planktonic and sessil dual species communities of them. Iron availability was used to measure differences in planktonic mixed growth of P. fluorescens and B. cereus. Under iron deficient condition, P. fluorescens prevented the vegetative growth of B. cereus. Dual biofilms were formed in a greater extent by B. cereus. B.cereus, being the primary surface colonizer, attached more effectively than P. fluorescens. Thus, the biofilms had a structure with a mid-layer composed of P. fluorescens, which was surrounded by two layers of B. cereus. The dominance of B. cereus in the outer layer of the dual biofilm is due to the constant supply of medium, and such flow may minimize the inhibitory factors formed by P. fluorescens. Also, the two specie dual biofilms had shown its weakened physical stability compared to that of the single species biofilms[13]. In other words, there is some antagonism relationship, or some similar effect between P. fluorescens and B. cereus in biofilms.
Do the microbes change their environment?
Binding to surface/Biofilm formation:
Hypothetically, the flow rate situated next to the substratum-liquid interface is insignificant. This area is coined with the term “hydrodynamic boundary layer” whose dimensions are inversely related to the water speed. Once a microbial cell has arrived at the hydrodynamic boundary layer, it can start the process of attacment [9].
The initial attachment of bacteria to a surface is often descrbied by the DLVO Theory proposed by Derjaquin and Landau and Verwey and Overbeek. Once a cell is inside the hydrodynamic boundary layer, it experiences an area of non-specific attraction about 10~20nm from the surface. This area is created due to the Van der Wall’s forces of attraction and electrostatic repulsion. At this stage, various properties of bacterial cell affects adhesion: Hydrophobic non-flagella appendages such as fimbrae help in cell attachment by overcoming the electrostatic repulsion barrier that is located between the cell and the substratum; Lipopolysaccharides and exo-polysaccharides, protein, and protein-carbohydrate interactions also play important roles in the process of cell attachment at this stage [9].
Overall microbial attachment involves a dynamic complex process. This can be affected by different variables such as flow rate, surface roughness and hydrophobicity, and the presence and properties of conditioning films. DUWS contains several features that encourage increase in bacterial contamination. Since the tubing of DUWS is composed of polyurethane and polyvinylchloride, it can serve as nutrient sources for the bacteria. Moreover, DUWS is faced with long periods of stagnation that will encourage biofilm formation as well [9].
When the cell is attched to a surface, cells start to grow quickly and form microcolony. Then secondary colonies, which are colonies that lack the ability to initially attach to a surface by themselvch to the primary colonisers [9].
A biofilm community can provide an ideal condition for the exchange of extrachromosomal DNA and plasmids. For example, plasmid recipient strains have been proven to demonstrate increased biofilm growth (penicillin binding proteins and IgA proteases) [9].
Lastly, when the biofilm is at the climax number of community, individual cells and parts of the biofilm can be lost from the surface due to the loss of nutrient, quorum sensing or shear forces. This occurs when a newly divided daughter cell is shed from the outermost layer of the biofilm. Detachment from physical process can result from erision or shear, rapid loss and abrasion. Even though high shear forces may reduce the biofilm, the laminar flow rates in DUWS are insufficient to result in a great loss of the biofilm structure (DUWS has the maximum flow rate of 100mL/min) [9].
Prevention and Solutions for Contaminated Dental Water Line
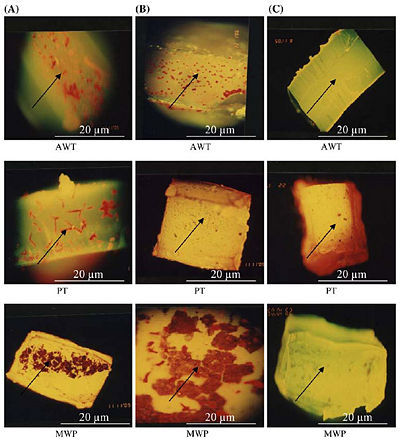
Non-chemical Treatment
DUWS engineering and redesigning: DUWS manufacturing is critical to achieve the improvements of water quality. Designing and evaluating DUWS is required to solve the problem of biofouling in the pipes. The length of the stagnant section can be reduced by redesigning the units and keep the water flow continuous. Also, the materials used could be re-evaluated to provide tubing that has less susceptibility to biofouling [10].
Flushing: Flushing the water line for several minutes before the first patient and for 20-30s between patients is recommended so that the number of bacteria in the water line can be decreased. However, this reduction is only temporary because the microorganisms will multiply back in a very short time. Therefore, an alternative strategy could be a flushing the dental unit at the end of the day and dry it overnight to reduce development of the biofilm [10].
Filtration: In-line 0.2 ㎛ membrane filters is used for the filtration of the dental unit water when they need to reduce the need for chemical treatment. The length of the DUWS does not matter too much, but the system beyond the filter will be susceptible to microbial contamination [10].
Ultraviolet Light (UV): As the water flows out over the UV lamp, the bacterial numbers will be reduced. It is mainly supplemented by filtration and/or alternating chemical treatment [10].
Physical Cleaning: This method uses sponges or balls by making them pass through the pipeline at high pressure in order to remove the biofilm and destroy biofouling. (This technique is not applicable to a current design of DUWS) [10].
Chemical Treatment
Many chemicals treatments are applied to treat the dental unit water. The products are used in the equipment to remove and kill biofilms. The common products consist of chemical compounds such as sodium hypochlorite, chlorine dioxide, peroxides, and citric acid. Other chemicals include electrochemically activated water for microbial control. Also, the use of biocides (a poisonous substance to living organisms) is one of the several proposed methods to reduce the contamination in the dental water line, such as removal of bioflim. When the NaOCl plus Phe combined chemical is added to the dental water lines, there has been an 85% to 95% decrease in bioflim formation and significant increase in bioflim removal from the surface [10].
Figure 9 above shows the epifuluoroscence microscopy of the biofilm in three different tubings, which are Air and Water Tubing (AWT), Patient Tubing (PT), and Main Water Pipe (MWP). Column (A) shows the thick colonization of biofilm without any treatment, column (B) shows a partially reduced biofilm as all biocides have been flushed through tubing samples, and column (C) shows nearly no biofilm after flushing with only NaOCl with Phe [10].
Current Research
First Topic: Effects of Various disinfectants.
Article: M. Özcan, Y. Kulak and E. Kazazoglu "The effect of disinfectant agents in eliminating the contamination of dental unit water." Journal of Oral Rehabilitation, 30; p.290–294.
Most serious spread of these microbial organisms arise from forming a community, or biofilm, which helps the organisms to survive washing or even drying up in these dental units. Since this can be detrimental to patients,dental offices have been trying to lower or even to get rid of these microbial organisms living in the dental unit waterline niche. Various methods including using electrolyzed acidic water and various chemical products are used to treat these waterlines, and this research paper focuses on two disinfectants (Bio® 2000 and Alpron®) and their effects on these waterlines. The unit for testing these waterlines’ condition was CFU (colony forming units) per mL. In order to effectively carry out the test, these two disinfectants were used according to the manufacturer’s instructions. The waterlines were treated on a daily basis at the end of each day by using the two disinfectants in the waterlines. Baseline, daily samples of 100 mL for the first week and the second samples for the week thereafter were plated on different agar plates. This resulted in: for Bio 2000, baseline had the mean value of CFU/mL of 36.25, first week and second week had 0; for Alpron, baseline had the mean value of CFU/mL of 760, first week had 0.75, and second week had 0. According to these results, the baseline had higher concentration of infection than the first week and second week of treatment for both Bio 2000 and Alpron. Moreover, since these two disinfectants were proved to be effective when used for more than two weeks, dental offices should be encouraged to use disinfectants to reduce the CFU/mL and detrimental health hazards [3].
Second Topic: Presence of Pseudomonas aeruginosa in the dental unit waterline systems.
Article: Al-Hiyasat AS, et al. "The presence of Pseudomonas aeruginosa in the dental unit waterline systems of teaching clincs." International Journal of Dental Hygiene 5 (2007): p.36-44.
It has been known from several former studies that there are pathogenic and opportunistic bacteria, such as Pseudomonas aeruginosa, Legionella pneumophila, and Staphylococcus aureus in DUW. This study was to examine the Pseudomonas aeruginosa contamination of DUW (Dental Unit water) at a Dental Teaching Center in Jordan. And to evaluate the extent of P. aeruginosa contamination, which is widely exposed to dental staff. Total of 30 dental units were used to collect water samples from three teaching clinics, conservative dentistry, periodontology, and prosthodontics. Samples were collected at the beginning of the working day, after 2 minutes of flushing, and at the midday from the air/water syringe, high-speed handpiece and water cup filler. Pseudomonas aeruginosa isolated from the dental unit water has shown to cause infection. Two patients’ mouth infection had same strain that has been isolated from the dental unit water system. The results show that at the beginning of the working day, 86.7% of the dental units examined were contaminated with P. aeruginosa. And flushing the dental water line for 2 minutes significantly decreased the amount of P. aeruginosa. Another information attained from the study was that the source of contamination in related to P. aeruginosa could be from patients’ mouths, meaning that the anti-retraction valves in dental units have failed to prevent the back flow of bacteria [14].
Third Topic: Legionella Contamination in Dental unit water.
Article: Zanetti F, et al."Water characteristics associated with the occurrence of Legionella Pneumophila in dental units." European Journal of Oral Sciences, 108; p.22-28
Legionella species are pathogenic micro-organisms which may be present in a variety of water systems. Majority of the Legionella are considered to be biofilm associated because they are more easily found in swab samples of biofilm than from flowing water. 101 water samples were taken from the incoming tap water, oral rinsing cup, air-water syringe, ultrasonic scaler, Legionella pneumophila was found in 22 samples. There was a greater frequency and concentration of L. Pnuemophila in the incoming water compared to the out-flowing water in the dental units. It was because of the complex structure of dental chair equipment. It helps the water to stagnate in the waterlines, leading to the formation of a biofilm. Through this study, it was also found that the organic matter enhances the occurrence of Legionella and the pH does not present a critical factor that regulates the growth of Legionella pneumophila. In addition, bacteria such as Psudomonas spp and Aeromonas spp have been tested to be capable of inhibiting Legionella. The primary route of infection of L. Pneumophila is via inhalation. Therefore, the presence of L. pneumophila in water used for dental treatments may threaten both patients and the dental team. In order to solve this problem, a disinfectant must be used to reduce the number of biofilm formation and also tubing system of the dental units need be tested against it [15].
Fourth Topic: Engineering of Dental Chair Unit.
Article: D.C. Coleman, et al."The Role of Manufacturers in Reducing biofilms in Dental Chair Waterlines." Journal of Dentistry, 35 (2007): p.701-711.
There is continuing research on how dental chair unit manufacturer would help to resolve the problem of biofilm formation in dental unit water. One of the improvements in manufacturing the dental chair unit to decrease the formation of bioflim in the dental unit water is, fully automated dental unit water disinfection systems that are developed for long-term effects. In prevention of back-siphonage of oral fluids into the water, improved anti-retraction devices are installed in the dental chair unit. Also, an air gap is installed to separate the dental unit waters in dental chair units from municipal mains water supplies. Researchers also found that the quality of water supply can be controlled in pre-treatment by using water softener and filtration, which removes the organic and inorganic materials in the dental unit water. They discovered that many of the large dental clinics are equipped with these systems in the dental chair unit. However, it is most important to perform periodic testing and maintenance to prevent bioflim formation [8].
Fifth Topic: Unusual Fungus in Dental Water Line.
Article: Porteous, Nuala B., et al. “Isolation of an Unusual Fungus in Treated Dental Unit Waterlines.” Journal of the American Dental Association 134. 7 (2003): p.853-858.
Many microbes reside in dental water line. Although most of such microbes are known to be non-pathogenic, heterotrophic bacteria, some of them like Legionella and Pseudonomas are opportunistic pathogens that are able to cause respiratory diseases such as pneumonia. The American Dental Association(ADA) recommends keeping colony-forming unit(CFU) level of non-surgical dental water lower than 200 CFU/ml.To examine the antibacterial effect of a continuous-use, stabilized chlorine dioxide compound, Porteous et al. organized a study where they used six dental units, all furnished with self-contained water system. Three of them were filled with a 1:10 dilution of continuous-use, stabilized chlorine dioxide compound, and the other three were used as control group. The authors sampled water from each of dental units weekly. The samples were properly cultured on R2A Agar for a week.Until the eighth week, bacterial colonies were isolated and observed from both treatment and control groups. In the ninth week, however, small, dark colonies which seemed to be fungal started to appear in a sample from one of the treated units. Porteous et al. also isolated colonies resembling such appearance from elsewhere in the same dental unit. Such fungus-like colonies were found in all the other treated units. After DNA sequencing process, the organism is diagnosed to be Exophiala mesophila. No fungus was isolated from any of dental units in control group which proposes that continuous use of stabilized chlorine dioxide compound may result in multiplication of a fungus in dental water line. [16]
Summary about the Dental Water Line niche
Dental chair units provide suitable environment for microbes to grow and to form biofilms. Biofilm formation in dental unit water has been understood by several theories, such as DLVO, and it has been researched through numerous experiments. According to the researches, there are interactions among the micro-organisms within biofilms and they may experience commensalism or antagonism. Those biofilms help microbes to survive well in the dental unit systems. The physical and chemical conditions of the dental unit waterlines differ from one to another, however, they maintain their stability most of the time. There are many bacteria, several fungi, and protozoa are found in the DUWs. Most common bacteria in dental waterlines are Mycobacterium spp., Legionelle spp., Pseudomonas spp., and Staphylococcus spp. Many studies have introduced effective methods to decontaminate the dental unit waterlines, and they can be divided into chemical and non-chemical treatment. Even though there have been many studies done in this field, there are still researches going on to improve the environment of DUWs.
References
(1) Kohno, S., Kawata T., et al. "Bactericidal Effects of Acidic Electrolyzed Water on the Dental Unit Waterline." Shinya Jpn J Infect Dis. Department of Orthodontics and Craniofacial Developmental Biology, Hiroshima University Graduate School of Biomedical Sciences. 57 (2004):52-54.29 Aug. 2008. <http://www.nih.go.jp/JJID/57/52.html>
(2) Kim, Eugene. D.D.S. Personal interview. 11 August 2008.
(3) Ozcan, M., Y. Kulak, and V. Kazazoglu. "The effect of disinfectant agents in eliminating the contamination of dental unit water." Journal of Oral Rehabilitation John Wiley & Sons, Inc. 30 (2003): 290–294. 29 Aug. 2008 <http://www3.interscience.wiley.com/cgi-bin/fulltext/118839807/HTMLSTART>
(4) Robert M, J. Barbeau, A. Prévost, and R. Charland. "Dental unit water lines: A propitious environment for bacterial colonization." Legionnaries' Desease. 29 Aug. 2008
(5) Souza-Gugelmin, Maria Cristina Monteiro, et al. "Microbial contamination in dental unit waterlines." Braz. Dent. Journal. Scielo.Br. 14, (2003). 29 Aug. 2008 <http://www.scielo.br/scielo.php?pid=S0103-64402003000100010&script=sci_arttext&tlng=en>
(6) Walker, James T., David J. Bradshaw, Allan M. Bennett, Martin R. Fulford, Michael V. Martin, and Philip D. Marsh. "Microbial Biofilm Formation and Contamination of Dental-Unit Water Systems in General Dental Practice." Appl Environ Microbiol 2000 August; 66(8): 3363-3367 <http://aem.asm.org/cgi/content/full/66/8/3363?view=long&pmid=10919792>
(7) Pankhurst, Caroline L., and N.W. Johnson. "Microbial contamination of dental unit waterlines: the scientific argument." International Dental Journal 48 (1998): 359–368. 28 August 2008 <http://www.ncbi.nlm.nih.gov/pubmed/9779119?ordinalpos=2&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum>
(8) Coleman, D.C., M.J. O'Donnell, A.C. Shore, J. Swan, R.J. Russell. "The Role of Manufacturers in Reducing Bioflims in Dental Chair Waterlines." Journal of Dentistry 35 (2007) 701-711. <http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T86-4P0N91R-1&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C000059602&_version=1&_urlVersion=0&_userid=4429&md5=69a82c61c2a64af5623cc97591fb522f >
(9) Walker, J.T., and P.D. Marsh. "A review of biofilms and their role in microbial contamination of dental unit water systems(DUWS)." International Biodeterioration & Biodegradation 54 (2004): 87 – 98. 28 August 2008 <http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6VG6-4CC2YY4-1&_user=4429&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_version=1&_urlVersion=0&_userid=4429&md5=158eca25a4e362f1e21594714380c638>
(10) Liaqat, I., and A.N. Sabri. "Effect of Biocides on Biofilm Bacteria from Dental Unit Water Lines." Current Microbiology 56 (2008): 619–624. 28 August 2008 <http://www.ncbi.nlm.nih.gov/pubmed/18322732>
(11) Walker, J.T., and P.D. Marsh. "Microbial biofilm formation in DUWS and their control using disinfectants." Journal of Dentistry 35 (2007): 721–730. 28 August 2008 <http://linkinghub.elsevier.com/retrieve/pii/S0300-5712(07)00132-7>
(12) Singh, T., and Coogan, M.M. "Isolation of pathogenic Legionella species and legionella-laden amoebae in dental unit waterlines" Journal of Hospital Infection 61 (2005): 257–262. 28 Aug. 2008 <http://linkinghub.elsevier.com/retrieve/pii/S0195-6701(05)00191-X>
(13) Simoes M., et al. "Antagonism between Bacillus cereus and Pseudomonas fluorescens in planktonic systems and in biofilms"Biofouling 24 (2008):5,339 — 349. 28 Aug. 2008 <http://www.informaworld.com/smpp/content~db=all?content=10.1080/08927010802239154>
(14) Al-Hiyasat AS, et al. "The presence of Pseudomonas aeruginosa in the dental unit waterline systems of teaching clincs." International Journal of Dental Hygiene 5 (2007): p.36-44. <http://www3.interscience.wiley.com/journal/118537317/abstract>
(15) Zanetti F, Stampi S, De Luca G, et al., "Water Characteristics associated with the occurrence of Legionella Pneumophila in dental units." European Journal of Oral Sciences. 108; p.22-28 <http://www.ncbi.nlm.nih.gov/pubmed/10706473>
(16) Porteous, Nuala B., et al. “Isolation of an Unusual Fungus in Treated Dental Unit Waterlines.” Journal of the American Dental Association 134. 7 (2003): 853-858. 29 August 2008 <http://jada.ada.org/cgi/content/full/134/7/853>.
(17) Shearer, BG. "Biofilm and the dental office." Journal of the American Dental Association 127. 2 (1996): 181-189. 29 August 2008 <http://jada.ada.org/cgi/reprint/127/2/181>
(18) Franco, F.F.S., D. Spratt, J.C. Leao and S.R. Porter. "Biofilm Formation and Control in Dental Unit Waterlines." Bioflims 2 (2005): 9-17.
(19) Sankaran, Neeraja. “Legionella pneumophila.” Microbes and People: an A-Z of Microorganisms in Our Lives. Phoenix: Oryx Press, 2000. 151-152.
(20) ---. “Mycobacterium.” Microbes and People: an A-Z of Microorganisms in Our Lives. Phoenix: Oryx Press, 2000. 169.
(21) ---. “Pseudomonas.” Microbes and People: an A-Z of Microorganisms in Our Lives. Phoenix: Oryx Press, 2000. 205-206.
(22) ---. “Pseudomonas aeruginosa.” Microbes and People: an A-Z of Microorganisms in Our Lives. Phoenix: Oryx Press, 2000. 205-206.
(23) ---. “Staphylococcus.” Microbes and People: an A-Z of Microorganisms in Our Lives. Phoenix: Oryx Press, 2000. 231-232.
(24) Atlas,R. M., et al. "Legionella Contamination of dental-unit waters." Applied Environmental Microbiology 61 (1995):1208-1213. 28 August 2008 <http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=167375>
Edited by Jane Choi, Bo R. Heo, Jinsoo Lee, Soh Yun Lee, and So Young Moon, students of Rachel Larsen