Dental Water Line
OUR WEB PAGE IS STILL UNDER CONSTRUCTION. THIS IS OUR WORK IN PROGRESS.
Introduction to Dental Water Line niche
INTRODUCTION HERE!!!
Description of Dental Water Line
Where located?
Modern dentists utilize various apparatuses in treating patients in their offices. These apparatuses include but are not limited to: dental chair units, triple syringe system, high speed handpieces, ultra sonic scalers and etc.[6] These dental units provide suitable conditions for dwelling for several forms of organisms including microbes and fungi. [5] One common condition that these dental units possess in providing the organisms a suitable condition to live in is that these units are always in contact with water. In order for these dental instruments to work properly with water, water-lining are installed through out the clinic from the city-water output to the primary filtering system to each dental chair unit, which has the secondary filtering system.[2] These units, however are left unused and their powers are off during the night, which aggravates the condition of the dental water line units ( or facilitates the growth of bacteria in these parts).
Physical Conditions?
What are the conditions in your niche? Temperature, pressure, pH, moisture, etc.
Temperature: Temperatures vary from one dental unit to another in most cases and also from one dental office to another. However, according to most of the references, it indicates that the temperature of this niche fall under the range of 23 to 37 degrees Celcius. [4] Some dental units have water heaters to heat the water so it would provide patients with more comfortable conditions. [8]
Moisture: There must be a constant flow of water since most dental unit waterlines require water.

pH: The pH of this niche is neutral. There have been studies where researchers used acidic electrolyzed in dental fields to effectively disinfect the contamination at the microbial level of dental waterlines, and these studies showed that the water before it was washed with acidic electrolyzed solution had a pH of 7. [1]
Water Flow: "In DUWS the flow is more likely to be laminar (60-100 ml min^-1 in comparison to the flow rate of a 1/2" (25 mm) diameter copper pipe of about 5000 ml min^-1) with long periods of stagnation, for example, overnight (16h) and at the weekend (64 h), that would favour microbial colozisation" [9]. DO NOT USE""
Influence by Adjacent Communities (if any)
Is your niche close to another niche or influenced by another community of organisms?
Many possibilities include: contamination or microbes getting transferred from one patient to dental units. When the patients are getting treated using the dental units, there is a possibility that dental units may collect the patient’s oral microbes and etc.
Another possibility may be the hands of dental staff since they deal with dental units and patients at the same time.
Conditions under which the environment changes
Do any of the physical conditions change? Are there chemicals, other organisms, nutrients, etc. that might change the community of your niche.
The physical conditions vary from one dental office to another, but for one particular dental office's waterline, their chemicals, water supply, nutrients, and organisms tend to stay constant most of the time.
However, by using disinfectants, organisms that live in this niche may be effected (killed, numbers reduced, etc). [3] For example, when the dental unit waterline is treated with chemicals or disinfectants, it is proven that the chemicals significantly alter the conditions of the waterline and reduce the number of contamination in the waterline. [3] For example, according to a research, using Alpron and Bio 2000 according to the manufacture’s instructions each clinical day reduced the infection level to zero CFU/mL after two weeks.[3] Another instance of altering the condition is to use electrolyzed acidic water to disinfect the contamination, which also resulted in reduced contamination of the water line. [1]
Discuss general information about biofilm becuase this part will be explained in-depth in Bacteria part of this page (Jinsoo).
Who lives in Dental Water Line?
Bacteria
Achromobacter xylosoxidans, Acidovorax defluvii, Acidovorax spp., Acinectobacter spp., Actinomyces spp., Aeromonas hydrophila, Alcaligenes dentifricans, Bacillus spp., Bacteroides spp., Caulobacter spp., Flavobacterium spp., Fusobacterium spp., Klebsiella pneumoniae, Lactobacillus spp., Legionella pneumophila, Legionella spp., Methylophilus spp., Micrococcus spp., Moraxella spp., Mycobacterium avium, Nocardia spp., Pasteurella spp., Porphyromonas gingivalis, Proteus vulgaris, Pseudomonas aeruginosa, Burkholderia cepacia, Sphigomonas paucimobilus, Sphingomonas spp., Streptococcus spp., Staphylococcus aureus, and Xanthomonas spp.[7].
Among bacteria that are listed above, there are four most commonly discussed bacteria within biofilms of Dental Water Line. They are Legionella pneumophila, Mycobacterium spp., Pseudomonas aeruginosa, and Staphylococcus spp..
Legionella pneumophila
Mycobacterium spp.
Pseudomonas aeruginosa
Staphylocococcus spp.

Fungi
Phoma spp., Penicillium spp., Cladosporium spp., Alternaris spp., and Scopulariopsis spp.[7].
Protozoa
Acanthamoeba spp., Cryptosporidium spp., Microsporidium spp., and Giardia spp.[7].
Do the microbes that are present interact with each other?
Describe any negative (competition) or positive (symbiosis) behavior
"The properties of biofilm cells have been shown to be distinct from their planktonic counterparts. There is evidence that gene expression may be regulated once a cell comes into contact with a surface. This up- and down- regulation of genes has been demonstrated in Pseudomonas aeruginosa with genes associated with alginate synthesis and in Staphylococcus aureus for genes encoding enzymes involved in glycolysis. In some organisms, up to 22% of genes were up-regulated in the biofilm state and 16% were down-regulated" [9].
"A biofilm community provides an ideal niche for the exchange of extrachromosomal DNA and plasmids. Cells in biofilms will exchange plasmids (conjugation) at a greater rate than cells in the planktonic phase, perhaps aided by the minimal shear and close cell-cell contact" [9].
"Biofilm cells, particularly P. aeruginosa, exhibit cell to cell signalling, which can play a role in attachment and detachment. At sufficient population densities these signal molecules, homoserine lactones in Gram-negative bacteria and peptides in Gram-positive bacteria, reach concentrations required for activation of genes involved in biofilm differentiation. In an analogous process Streptococcus cristatus was able to prevent attachment of Porphyromonas gingivalis to a dental plaque biofilm. Aeromonas hydrophila forms biofilms in water systems. Mutants of A. hydrophila in cell-cell communication were impaired in their ability to form biofilms as has been demonstrated in other strains" [9].
"These biofilms can have a diverse composition and the constituent microorganisms can engage in a range of interactions to form a structured and functionally- organised microbial community. The interactions would involve examples of co-operation and antagonism including competition and predation due to bacteriovores, such as free living protozoa that have been found to be 300 times more numerous in DUWs than in tap water, and bacteriophage (bacterial viruses) that may attack specific bacterial species in the biofilm" [11].
"Free-living amoebae represent an important reservoir of legionella and other pathogens and may, while encysted, protect the internalized micro-organism from chlorine and other biocides. The amoebae produce vesicles filled with the pathogens, increasing their transmission potential" [12].
"In conclusion, this study shows significant differences in the behaviour and composition of planktonic and sessile dual species communities of B. cereus and P. fluorescens. Planktonic mixed growth of P. fluorescens and B. cereus was regulated by iron availability. Under Fe-deficiency, P. fluorescens inhibited the vegetative growth of B. cereus. This inhibition was apparently related to the production of Fechelating molecules. Dual biofilms were colonised to a higher extent by B. cereus, the primary surface coloniser, which attached to SS more effectively than P. fluorescens. These biofilms had a stratified structure with a middle layer composed mostly of P. fluorescens, surrounded by two layers where B. cereus was the predominant species. The prevalence of B. cereus in the outermost layer of dual biofilms seems to be due to the constant supply of fresh medium, which is needed for the bioreactor system. Such a constant flow minimises the concentration of inhibitory factors produced by P. fluorescens within the bioreactor. Furthermore, the two species biofilms had decreased physical stability relative to that of the single species biofilms. Further studies are being carried out to characterise the exact chemical nature of the B. cereus antagonist molecule produced by P. fluorescens" [13].
Do the microbes change their environment?
Do they alter pH, attach to surfaces, secrete anything, etc. etc.
"In theory, the flow velocity immediately adjacent to the substratum-liquid interfce is negligible. This zone of insignificant flow is termed the hydrodynamic boundary layer whose dimensions are inversely related to the water velocity. Once a microbial cell has reached the hydrodynamic boudary layer then the processes that are involved in attachment can begin" [9].
"The initial attachment of bacteria to a surface can be described using the theory proposed by Derjaquin and Landau and Verwey and Overbeek and known as the DLVO Theory. As a cell penetrates the hydrodynamic boundary layer it experiences an area of overall non-specific attraction about 10-20 nm from the surface. This area is due to the interplay of van der Wall's forces of attraction and electrostatic repulsion; theses forces act on the whole cell body and are long range, weak, reversible interactions...It is at this stage that the specific properties of the bacterial cell begin to influence adhesion. Hydrophobic non-flagella appendages such as fimbrae play a role in cell attchment by overcoming the electrostatic repulsion barrier that exists between the cell and the substratum. Lipopolysaccharides and exo-polysaccharides also play a role in the attachment process. This stage involves both protein and protein-carbohydrate interactions (lectin)" [9].
"Microbial attachment is a dynamic complex process, which can be affected by many varialbes including flow rate, surface roughness and hydrophobicity, and the presence and properties of conditioning films. Several features of DUWS may encourage bacterial contamination. The tubing in DUWS is composed of polyurethane and polyvinylchloride (PVS). This is a good substrate for biofilm formation because of the hardeners and additives that can be used by the bacteria as nutirent sources. In addition, DUWS suffer from long periods of stagnation that will also encourage biofilm proliferation" [9].
"Plasmid recipient strains have been shown to demonstrate increased biofilm growth. Examples include the transfer of penicillin binding proteins (PBP) and IgA proteases" [9].
"As the biofilm gains the status of a climax community, individual cells and parts of the biofilm may be lost from the heterogeneous mass on the surface as a result of nutrient depletion, quorum sensing or shear forces. This can be in the form of newly divided daughter cells being shed from the outermost region of the biofilm. Detachment due to physical processes can be because of erosion or shear (continual removal), sloughing (rapid loss) and abrasion (due to collision)...Whilst very high shear forces may play a role in diminishing the biofilm, the laminar flow rates found in DUWS are unlikely to result in any great loss of the biofilm structure even when the DUWS is turned to maximum flow rate of 100 ml min^-1" [9].
Do the microbes carry out any metabolism that affects their environment?
Do they ferment sugars to produce acid, break down large molecules, fix nitrogen, etc. etc.
Prevention and Solutions for Contaminated Dental Water Line
MAYBE PREVENTION HERE???
"Several methods of decreasing the level of contamination in DUWL have been proposed, one of these being the use of biocides (ie, compounds with lethal activity against living organisms). These are employed to a variable extent to remove the biofilm" [10].
"The combined effect of NaOCl plus Phe resulted in an 85% to 95% decrease in biofilm viability and significant removal of biofilm from the surface" [10].
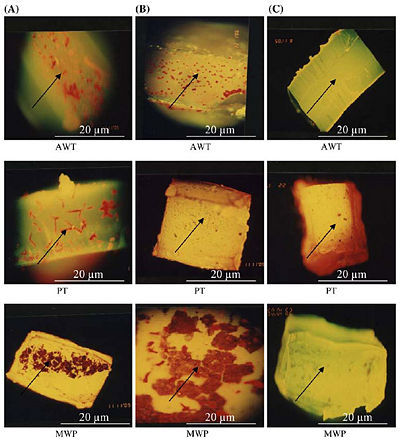
Figure 3 on the right, shows the epifuluoroscence microscopy of the biofilm in three different tubings. AWT stands for Air and Water Tubing, PT stands for Patient Tubing, and MWP stands for Main Water Pipe. Column (A) shows the thick colonization of biofilm without any treatment, column (B) shows a partially reduced biofilm as all biocides have been flushed through tubing samples, and column (C) shows nearly no biofilm after flushing with only NaOCl with Phe [10].
Current Research
First Topic: Effects of Various disinfectants.
Article: "The effect of disinfectant agents in eliminating the contamination of dental unit water." Journal of Oral Rehabilitation, 30; p290–294. [3]
Most serious spread of these microbial organisms arise from forming a community, or biofilm, which helps the organisms to survive washing or even drying up in these dental units. Since this can be detrimental to patients[3],dental offices have been trying to lower or even to get rid of these microbial organisms living in the dental unit waterline niche. Even without these detrimental factors, the ADA recommends maximum permissible levels of less than 200 CFU/mL [3]. Various methods including using electrolyzed acidic water and various chemical products are used to treat these waterlines, and this research paper focuses on two disinfectants (Bio® 2000 and Alpron®) and their effects on these waterlines.[3] The unit for testing these waterlines’ condition was CFU (colony forming units) per mL[3]. In order to effectively carry out the test, these two disinfectants were used according to the manufacturer’s instructions. The waterlines were treated on a daily basis at the end of each day by using the two disinfectants in the waterlines. Baseline, daily samples of 100 mL for the first week and the second samples for the week thereafter were plated on different agar plates. This resulted in: for Bio 2000, baseline had the mean value of CFU/mL of 36.25, first week and second week had 0; for Alpron, baseline had the mean value of CFU/mL of 760, first week had 0.75, and second week had 0.[3] According to these results, the baseline had higher concentration of infection than the first week and second week of treatment for both Bio 2000 and Alpron. Moreover, since these two disinfectants were proved to be effective when used for more than two weeks, dental offices should be encouraged to use disinfectants to reduce the CFU/mL and detrimental health hazards.
Summary about the Dental Water Line niche
SUMMARY HERE!!!
References
(2) Kim, Eugene. D.D.S. has given certain comments about how the dental clinic is constructed and how the water lining is installed. (TO BE EDITED).
(7) Pankhurst et al, 1998; D. Spratt (TO BE EDITED)
(9) J.T. Walker, P.D. Marsh. A review of biofilms and their role in microbial contamination of dental unit water systems(DUWS). (TO BE EDITED)
(10) I. Liaqat, A.N. Sabri. Effect of Biocides on Biofilm Bacteria from Dental Unit Water Lines. (To be edited)
(11) J.T. Walker, P.D. Marsh. Microbial biofilm formation in DUWS and their control using disinfectants. (To be edited)
(12) T. Singh, M.M. Coogan. Isolation of pathogenic Legionella species and legionella-laden amoebae in dental unit waterlines. (to be edited)
(13) Manuel Simoes, Lucia Simoes, Maria O. Pereira, and Maria J, Vieira. Antagonism between Bacillus cereus and Pseudomonas fluorescens in planktonic systems and in biofilms (to be edited)
(14) AS Al-Hiyasat, SY Ma'ayeh, MY Hindiyeh, and YS Khader. The presence of Pseudomonas aeruginosa in the dental unit waterline systems of teaching clinics. (to be edited)
Edited by Jane Choi, Bo R. Heo, Jinsoo Lee, Soh Yun Lee, and So Young Moon, students of Rachel Larsen
