Gastritis and Peptic Ulcer Disease Caused by Helicobacter pylori
Introduction
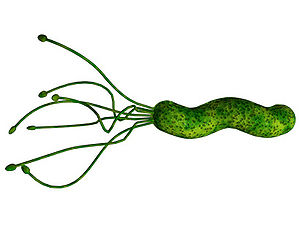
Helicobacter pylori is a Gram-negative, helix-shaped bacterium that is about 3 micrometers long with a diameter of 0.5 micrometers. H. pylori is a microaerophilic bacterium which means that it requires oxygen to function. However, H. pylori requires much lower concentrations of oxygen than those found in our atmosphere. This bacterium contains a hydrogenase which it can use to obtain energy by oxidizing molecular hydrogen (in the form of H2) produced by intestinal bacteria. H. pylori also produces oxidase, catalase, and urease. It has an outer-membrane consisting of phospholipids and lipopolysaccharide which are characteristic of typical Gram-negative bacteria. H. pylori bacteria are known to inhabit various areas of the stomach and duodenum. Infections caused by this bacteria lead to chronic inflammation in the stomach lining (Gastritis). H. pylori infections are also strongly associated to the development of gastric ulcers and even stomach cancer. Although H. pylori is known to cause several problems, over 80% of individuals infected with this bacterium show no symptoms. Over half of all people in the world are thought to harbor this bacterium in their upper gastrointestinal tract.
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Introduce the topic of your paper. What microorganisms are of interest? Habitat? Applications for medicine and/or environment?
Pathogenesis
.
In order to colonize the stomach H. pylori must survive the acidic pH of its environment. It burrows into the mucus lining that coats the stomach. An H. pylori bacterium has flagella and uses them to move through the stomach lumen and drill into the mucoid lining of the stomach.[1] H. pylori must insert itself near the stomach's epithelial cell layer which is this bacterium’s niche. H. pylori can be found deep in the mucoid lining of the stomach. Mucous is continuously secreted by mucous cells and removed on the luminal side. To avoid being carried into the lumen by the newly formed mucous, H. pylori senses the pH gradient within the mucus layer by chemotaxis and swims away from the acidic contents of the lumen towards the more neutral pH environment of the epithelial cell surface.[2] H. pylori can also be present on the inner surface of the epithelial cells of the stomach. Sometimes the bacterium is even found inside the epithelial cells.[3] H. pylori produces adhesins that bind to membrane-associated lipids and carbohydrates which helps it to adhere to the epithelial cells.[4] The bacterium also produces great quantities of urease, and enzyme located inside and outside of the cell. This enzyme is capable of breaking down urea that is secreted into the stomach. It breaks down the urea into carbon dioxide and ammonia. The ammonia is converted into an ammonium ion by accepting a hydrogen ion from self-ionized water. The left-over hydroxyl ions then react with the carbon dioxide to produce bicarbonate which neutralizes gastric acid. Therefore, H. pylori is dependent upon urease for its survival in the acidic environment found in the stomach. Without the urease enzyme the bacterium would almost inevitably die. The ammonia that is a byproduct of the urease reaction is toxic to epithelial cells of the stomach. H. pylori also produces protease (an enzyme that breaks down proteins), vacuolating cytotoxin A (VacA), and phospholipases (enzymes that hydrolyze phospholipids into fatty acids and other lipophilic substances). All of these products are damaging to the epithelial cells of the stomach.[5]
When H. pylori colonizes the stomach it often results in chronic gastritis, an inflammation of the stomach lining. Stomach and duodenal ulcers occur when the inflammation allows the acid and pepsin in the stomach lumen to overpower the mechanisms that protect the stomach and duodenal mucosa from these substances. The location of the chronic gastritis (which occurs at the site of H. pylori colonization) dictates the type of ulcer that subsequently develops.[6] The amount of acid within the stomach lumen has an effect on the colonization of patterns of the bacterium H. pylori. It will therefore, in the end, establish the location at which the gastric or duodenal ulcer will form. For example, in people that produce large amounts of acid, H. pylori will colonize the pyloric antrum of the stomach to avoid the parietal cells in the corpus of the stomach that secrete acid.[7]
The inflammatory response to the bacteria induces G cells in the antrum to secrete the hormone gastrin. Gastrin travels through the bloodstream to the corpus of the stomach.[8] Gastrin excites the parietal cells in the corpus to release even more acid into the stomach lumen. Persistently increased gastrin levels eventually cause the number of parietal cells to also increase, further increasing the amount of acid secreted.[9] The increased amount of acid damages the duodenum and may consequently result in the formation of duodenal ulcers. Gastric ulcers on the other hand are often linked with reduced or normal levels of acid production. This suggests that the mechanisms that protect the gastric mucosa are faulty in that individual.[9] H. pylori capitalizes on this defect and begins to colonize the corpus of the stomach where the parietal cells are located. The chronic inflammation caused by the colonization of this bacterium causes further reduction in the stomach’s acid production, causing atrophy of the stomach lining. The deterioration of the stomach’s lining may lead to future gastric ulceration and even an increased risk for stomach cancer.[10]
There are some strains of H. pylori that are capable of triggering a greater inflammatory response in the stomach of its host. This strain carries the cag pathogenicity island (cag PAI). Over half of the H. pylori strains in Western countries are thought to carry the cag PAI. [11] Not only do these strains create a stronger inflammatory response in the stomach but they also create a greater risk for developing ulcers or cancer than the strains lacking the cag PAI.[7] The cag PAI expresses a type IV secretion system after the attachment of the bacterium to the epithelial cells of the stomach. This system inserts peptidoglycan from the bacterial cell wall into the epithelial cells. This peptidoglycan acts as an inflammatory inducing agent within epithelial cells. It is recognized by the cytoplasmic immune sensor Nod1 that stimulates the expression of cytokines which promote inflammation.[12]
Section 2
Include some current research, with at least one figure showing data.
Section 3
Include some current research, with at least one figure showing data.
Conclusion
Overall text length at least 3,000 words, with at least 3 figures.
References
1. Ottemann KM, Lowenthal AC (April 2002). "Helicobacter pylori uses motility for initial colonization and to attain robust infection". Infect. Immun. 70 (4): 1984–90. doi:10.1128/IAI.70.4.1984-1990.2002. PMID 11895962. PMC 127824. http://iai.asm.org/cgi/pmidlookup?view=long&pmid=11895962.
2. Schreiber S, Konradt M, Groll C, et al. (April 2004). "The spatial orientation of Helicobacter pylori in the gastric mucus". Proc. Natl. Acad. Sci. U.S.A. 101 (14): 5024–9. doi:10.1073/pnas.0308386101. PMID 15044704.
3. Petersen AM, Krogfelt KA (May 2003). "Helicobacter pylori: an invading microorganism? A review". FEMS Immunol. Med. Microbiol. 36 (3): 117–26. doi:10.1016/S0928-8244(03)00020-8. PMID 12738380.
4. Ilver D, Arnqvist A, Ogren J, et al. (January 1998). "Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging". Science (journal) 279 (5349): 373–7. PMID 9430586.
5. Smoot DT (December 1997). "How does Helicobacter pylori cause mucosal damage? Direct mechanisms". Gastroenterology 113 (6 Suppl): S31–4; discussion S50. PMID 9394757.
6. Dixon MF (February 2000). "Patterns of inflammation linked to ulcer disease". Baillieres Best Pract Res Clin Gastroenterol 14 (1): 27–40. doi:10.1053/bega.1999.0057. PMID 10749087.
7. Kusters JG, van Vliet AH, Kuipers EJ (July 2006). "Pathogenesis of Helicobacter pylori infection". Clin Microbiol Rev 19 (3): 449–90. doi:10.1128/CMR.00054-05. PMID 16847081.
8. Blaser MJ, Atherton JC (February 2004). "Helicobacter pylori persistence: biology and disease". J. Clin. Invest. 113 (3): 321–33. doi:10.1172/JCI20925. PMID 14755326.
9. Schubert ML, Peura DA (June 2008). "Control of gastric acid secretion in health and disease". Gastroenterology 134 (7): 1842–60. doi:10.1053/j.gastro.2008.05.021. PMID 18474247.
10. Suerbaum S, Michetti P (October 2002). "Helicobacter pylori infection". N. Engl. J. Med. 347 (15): 1175–86. doi:10.1056/NEJMra020542. PMID 12374879.
Broxmeyer, L., Sosnowska, D., Miltner, E., Chacon, O., Wagner, D., McGarvey, J., Barletta, R.G., and Bermudez, L.E. "Killing of Mycobacterium avium and Mycobacterium tuberculosis by a Mycobacteriophage Delivered by a Nonvirulent Mycobacterium: A Model for Phage Therapy of Intracellular Bacterial Pathogens". The Journal of Infectious Diseases. 2002. Volume 186, Number 8. p. 1155-1160.
Clark, J.R. and March, J.B. "Bacteriophages and biotechnology: vaccines, gene therapy and antibacterials". TRENDS in Biotechnology. 2006. Volume 24, Number 5. p. 212-218.
Inal, J.M. "Phage Therapy: a Reappraisal of Bacteriophages as Antibiotics". Archivum Immunologiae et Therapiae Experimentalis. 2003. Volume 51. p. 237-244.