Helicobacter pylori as a causative agent of Gastric Cancer: Difference between revisions
| Line 23: | Line 23: | ||
==<i>Helicobacter pylori</i> Description and Genome== | ==<i>Helicobacter pylori</i> Description and Genome== | ||
Helicobacter pylori is a gram-negative proteobacteria that adheres extracellularly to the epithelial cells found within the human stomach [4] [figure 2]. Acquired during infancy [4], H. pylori is typically contracted through contaminated water and food or through oral contact, especially in situations of poverty and densely populated areas [1]. The bacterium is a microaerophile and thrives in extremely acidic conditions with low levels of oxygen, such as the environment of the gastrointestinal tract [3]. The bacterium is able to survive in this type of environment through its production and use of hydrogenase that is coupled with aerobic respiration [6]. | |||
<br><br> | |||
The bacterium commonly presents morphologically as a curved rod with flagella; flagella are crucial to H. pylori survival as they allow for motility through the gastric mucosal layer in order to infect the host cells [7]. Interestingly, H. pylori in the form of cocci have been observed after treatment with antibiotics [4]. It is not known as of yet whether these coccoidal structures are of significance pathogenically. | |||
<br><br> | |||
Evidence has been found to suggest that H. pylori had begun to colonize within epithelial cells in humans as far back as 60,000 years ago in East Africa [8]. Mutations in the bacterial genome are distinct and indicative of human migration patterns [8]. As of 2011, seven full genomes were sequenced, which are composed of 1.6 megabases each, and 1500 open reading frames [4]. Mutations between species have been observed in 20-30% of the genome dependent on bacterial strain, which is indicative of a high mutation rate as well as high recombination frequency [4]. | |||
<br><br> | |||
Although about 80% of H. pylori colonization is benign and asymptomatic, the remaining percentage of the population infected with this bacterium experience some sort of abnormal tissue growth overtime, with gastric tumors growing in less than 2% [4]. In varying degrees, H. pylori possess the capability to induce gastritis and low levels of gastric acid production [2]. Depletion over time of the organism within the stomach is uncommon; however, some cases have shown loss of H. pylori in situations where stomach mucous becomes unfavorable for colonization such as during metaplasia [4]. | |||
<br><br> | |||
Helicobacter pylori utilizes a variety of adhesion mechanisms in order to survive within the human gastrointestinal tract. Of particular interest is the cytoxin associated gene (CAG) pathogen island, which translates a variety of proteins that assist in depositing pathogenic products into host cells [figure 3] [4]. Other compounds of note are blood group antigen binding adhesin (BabA) and sialic acid binding adhesin (SabA), both of which are responsible in facilitating H. pylori binding to epithelial cells [4]. | |||
<br><br> | |||
H. pylori facilitates survival within its host through the utilization of a urease enzyme which creates an extremely acid environment (pH 1-2) for the bacterium [4]. H. pylori flagella are particularly useful to the organism in facilitation of motion through the mucous layer that covers epithelial cells within the stomach [4]. | |||
<br><br> | |||
Typically found as adhering extracellularly to epithelial cells, H. pylori has been found in some cases to be implicated in antibiotic resistance when observed intracellularly, particularly in cancers [9]. | |||
==Gastric Cancer== | ==Gastric Cancer== | ||
Revision as of 17:52, 22 April 2015
Introduction
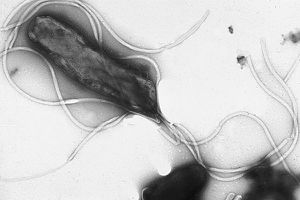
Helicobacter pylori [figure 1] is an important bacterium that colonizes in the stomachs of over two-thirds of the human population, and is especially prevalent in developing countries [1]. Although latent in the majority of infected persons, inflammation caused by H. pylori has been found to be the main factor in gastritis, peptic ulcers, and in some cases gastric cancer [2]. In fact, three-fourths of all stomach cancer cases are caused by H. pylori colonization [3]. This form of carcinogenesis is the second most prevalent cause of deaths caused by cancer, with a death count of about 738,000 people in 2008 [1].
In fact, H. pylori is currently the only bacterium in existence to be categorized as a carcinogen by the World Health Organization International Agency for Research on Cancer [3]. Colonization by this bacterium is a risk factor and causative agent for non-cardia gastric cancer, which affects all areas of the stomach except for the coronal area of the stomach near its juncture with the esophagus [1]. In addition, infection with H. pylori is an agent for gastric mucosa-associated lymphoid tissue lymphoma (MALT) [1].
There are a variety of mechanisms utilized by H. pylori that are thought to promote gastric carcinoma; overall, H. pylori causes oxidative and nitrosative stress on epithelial gastric cells, which results in both cellular and nucleic acid damage, leading to carcinogenesis [4].
Evidence for H. pylori as a causative agent of gastric adenocarcinoma is abundant, yet is only a risk factor for non-cardia gastric cancer. In fact, the risk of presenting with stomach cancer is six times greater for patients infected with H. pylori than those who are uninfected [5].
Simultaneously, H. pylori infection has been found to lessen the risk of other cancers, in particular carcinoma of gastric cardia cancer and esophageal adenocarcinoma [1]. It is not known as of yet the reasoning for why H. pylori causes cancer in the caudal area of the stomach yet not in the cranial section.
Helicobacter pylori Description and Genome
Helicobacter pylori is a gram-negative proteobacteria that adheres extracellularly to the epithelial cells found within the human stomach [4] [figure 2]. Acquired during infancy [4], H. pylori is typically contracted through contaminated water and food or through oral contact, especially in situations of poverty and densely populated areas [1]. The bacterium is a microaerophile and thrives in extremely acidic conditions with low levels of oxygen, such as the environment of the gastrointestinal tract [3]. The bacterium is able to survive in this type of environment through its production and use of hydrogenase that is coupled with aerobic respiration [6].
The bacterium commonly presents morphologically as a curved rod with flagella; flagella are crucial to H. pylori survival as they allow for motility through the gastric mucosal layer in order to infect the host cells [7]. Interestingly, H. pylori in the form of cocci have been observed after treatment with antibiotics [4]. It is not known as of yet whether these coccoidal structures are of significance pathogenically.
Evidence has been found to suggest that H. pylori had begun to colonize within epithelial cells in humans as far back as 60,000 years ago in East Africa [8]. Mutations in the bacterial genome are distinct and indicative of human migration patterns [8]. As of 2011, seven full genomes were sequenced, which are composed of 1.6 megabases each, and 1500 open reading frames [4]. Mutations between species have been observed in 20-30% of the genome dependent on bacterial strain, which is indicative of a high mutation rate as well as high recombination frequency [4].
Although about 80% of H. pylori colonization is benign and asymptomatic, the remaining percentage of the population infected with this bacterium experience some sort of abnormal tissue growth overtime, with gastric tumors growing in less than 2% [4]. In varying degrees, H. pylori possess the capability to induce gastritis and low levels of gastric acid production [2]. Depletion over time of the organism within the stomach is uncommon; however, some cases have shown loss of H. pylori in situations where stomach mucous becomes unfavorable for colonization such as during metaplasia [4].
Helicobacter pylori utilizes a variety of adhesion mechanisms in order to survive within the human gastrointestinal tract. Of particular interest is the cytoxin associated gene (CAG) pathogen island, which translates a variety of proteins that assist in depositing pathogenic products into host cells [figure 3] [4]. Other compounds of note are blood group antigen binding adhesin (BabA) and sialic acid binding adhesin (SabA), both of which are responsible in facilitating H. pylori binding to epithelial cells [4].
H. pylori facilitates survival within its host through the utilization of a urease enzyme which creates an extremely acid environment (pH 1-2) for the bacterium [4]. H. pylori flagella are particularly useful to the organism in facilitation of motion through the mucous layer that covers epithelial cells within the stomach [4].
Typically found as adhering extracellularly to epithelial cells, H. pylori has been found in some cases to be implicated in antibiotic resistance when observed intracellularly, particularly in cancers [9].
Gastric Cancer
Symptoms and Treatment
Process of Infection
Adhesin
DNA Repair Pathways
Inflammation
Future Work
References
[1] Helicobacter pylori and Cancer. (2013, September 5). Retrieved April 17, 2015, from http://www.cancer.gov/cancertopics/causes-prevention/risk/infectious-agents/h-pylori-fact-sheet
[2] Machado, A. M. D., Figueiredo, C., Touati, E., Máximo, V., Sousa, S., Michel, V., & Rasmussen, L. J. (2009). Helicobacter pylori infection induces genetic instability of nuclear and mitochondrial DNA in gastric cells. Clinical Cancer Research, 15(9), 2995-3002.
[3] Suganuma, M., Watanabe, T., Yamaguchi, K., Takahashi, A., & Fujiki, H. (2012). Human gastric cancer development with TNF-α-inducing protein secreted from Helicobacter pylori. Cancer letters, 322(2), 133-138.
[4] Kim, S. S., Ruiz, V. E., Carroll, J. D., & Moss, S. F. (2011). Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer letters ,305(2), 228-238.
[5] Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: A combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001; 49(3):347–353.
[6] Maier, R. J., Fu, C., Gilbert, J., Moshiri, F., Olson, J., & Plaut, A. G. (1996). Hydrogen uptake hydrogenase in Helicobacter pylori. FEMS microbiology letters, 141(1), 71-76.
[7] Ottemann, K. M., & Lowenthal, A. C. (2002). Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infection and immunity, 70(4), 1984-1990.
[8] D. Falush, T. Wirth, B. Linz, et. al., Traces of human migrations in Helicobacter pylori populations, Science 299 (2003).
[9] A. Dubois, T. Boren, Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell Microbiol. 9 (2007).
[10] Mayo Clinic. (n.d.). Stomach Cancer. Retrieved April 17, 2015, from http://www.mayoclinic.org/diseases-conditions/stomach-cancer/basics/definition/con-20038197
[11] Stomach Cancer. (n.d.). Retrieved April 20, 2015, from http://www.mayoclinic.org/diseases-conditions/stomach-cancer/basics/treatment/con-20038197
[12] National Cancer Institute. (n.d.). Stomach Cancer: MedlinePlus. Retrieved April 17, 2015, from http://www.nlm.nih.gov/medlineplus/stomachcancer.html
[13] R.M. Peek Jr., M.J. Blaser, Helicobacter pylori and gastrointestinal tract adenocarcinomas, Nature 2 (2002) 28-37
[14] Bagnoli F, Buti L, Tompkins L, Covacci A, Amieva MR. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proceedings of the National Academy of Science USA 2005; 102(45):16339–16344.
[15] André AR, Ferreira MV, Mota RM, et al. Gastric adenocarcinoma and Helicobacter pylori: Correlation with p53 mutation and p27 immunoexpression. Cancer Epidemiology 2010; 34(5):618–625.
[16] Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. Journal of the National Cancer Institute 2012; 104(6):488-492.
Edited by Alexandra Kruse, student of Joan Slonczewski for BIOL 238 Microbiology, 2015, Kenyon College.