Helicobacter pylori as a causative agent of Gastric Cancer
Introduction
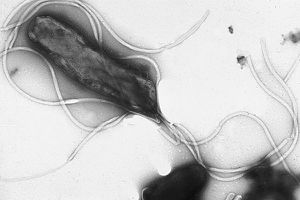
Helicobacter pylori [figure 1] is an important bacterium that colonizes in the stomachs of over two-thirds of the human population, and is especially prevalent in developing countries [1]. Although latent in the majority of infected persons, inflammation caused by H. pylori has been found to be the main factor in gastritis, peptic ulcers, and in some cases gastric cancer [2]. In fact, three-fourths of all stomach cancer cases are caused by H. pylori colonization [3]. This form of carcinogenesis is the second most prevalent cause of deaths caused by cancer, with a death count of about 738,000 people in 2008 [1].
In fact, H. pylori is currently the only bacterium in existence to be categorized as a carcinogen by the World Health Organization International Agency for Research on Cancer [3]. Colonization by this bacterium is a risk factor and causative agent for non-cardia gastric cancer, which affects all areas of the stomach except for the coronal area of the stomach near its juncture with the esophagus [1]. In addition, infection with H. pylori is an agent for gastric mucosa-associated lymphoid tissue lymphoma (MALT) [1].
There are a variety of mechanisms utilized by H. pylori that are thought to promote gastric carcinoma; overall, H. pylori causes oxidative and nitrosative stress on epithelial gastric cells, which results in both cellular and nucleic acid damage, leading to carcinogenesis [4].
Evidence for H. pylori as a causative agent of gastric adenocarcinoma is abundant, yet is only a risk factor for non-cardia gastric cancer. In fact, the risk of presenting with stomach cancer is six times greater for patients infected with H. pylori than those who are uninfected [5].
Simultaneously, H. pylori infection has been found to lessen the risk of other cancers, in particular carcinoma of gastric cardia cancer and esophageal adenocarcinoma [1]. It is not known as of yet the reasoning for why H. pylori causes cancer in the caudal area of the stomach yet not in the cranial section.
Helicobacter pylori Description and Genome
Gastric Cancer
Symptoms and Treatment
Process of Infection
Adhesin
DNA Repair Pathways
Inflammation
Future Work
References
[1] Helicobacter pylori and Cancer. (2013, September 5). Retrieved April 17, 2015, from http://www.cancer.gov/cancertopics/causes-prevention/risk/infectious-agents/h-pylori-fact-sheet
[2] Machado, A. M. D., Figueiredo, C., Touati, E., Máximo, V., Sousa, S., Michel, V., & Rasmussen, L. J. (2009). Helicobacter pylori infection induces genetic instability of nuclear and mitochondrial DNA in gastric cells. Clinical Cancer Research, 15(9), 2995-3002.
[3] Suganuma, M., Watanabe, T., Yamaguchi, K., Takahashi, A., & Fujiki, H. (2012). Human gastric cancer development with TNF-α-inducing protein secreted from Helicobacter pylori. Cancer letters, 322(2), 133-138.
[4] Kim, S. S., Ruiz, V. E., Carroll, J. D., & Moss, S. F. (2011). Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer letters ,305(2), 228-238.
[5] Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: A combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001; 49(3):347–353.
[6] Maier, R. J., Fu, C., Gilbert, J., Moshiri, F., Olson, J., & Plaut, A. G. (1996). Hydrogen uptake hydrogenase in Helicobacter pylori. FEMS microbiology letters, 141(1), 71-76.
[7] Ottemann, K. M., & Lowenthal, A. C. (2002). Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infection and immunity, 70(4), 1984-1990.
[8] D. Falush, T. Wirth, B. Linz, et. al., Traces of human migrations in Helicobacter pylori populations, Science 299 (2003).
[9] A. Dubois, T. Boren, Helicobacter pylori is invasive and it may be a facultative intracellular organism. Cell Microbiol. 9 (2007).
[10] Mayo Clinic. (n.d.). Stomach Cancer. Retrieved April 17, 2015, from http://www.mayoclinic.org/diseases-conditions/stomach-cancer/basics/definition/con-20038197
[11] Stomach Cancer. (n.d.). Retrieved April 20, 2015, from http://www.mayoclinic.org/diseases-conditions/stomach-cancer/basics/treatment/con-20038197
[12] National Cancer Institute. (n.d.). Stomach Cancer: MedlinePlus. Retrieved April 17, 2015, from http://www.nlm.nih.gov/medlineplus/stomachcancer.html
[13] R.M. Peek Jr., M.J. Blaser, Helicobacter pylori and gastrointestinal tract adenocarcinomas, Nature 2 (2002) 28-37
[14] Bagnoli F, Buti L, Tompkins L, Covacci A, Amieva MR. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proceedings of the National Academy of Science USA 2005; 102(45):16339–16344.
[15] André AR, Ferreira MV, Mota RM, et al. Gastric adenocarcinoma and Helicobacter pylori: Correlation with p53 mutation and p27 immunoexpression. Cancer Epidemiology 2010; 34(5):618–625.
[16] Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. Journal of the National Cancer Institute 2012; 104(6):488-492.
Edited by Alexandra Kruse, student of Joan Slonczewski for BIOL 238 Microbiology, 2015, Kenyon College.