Pneumococcal meningitis and the role of Streptococcus pneumoniae: Difference between revisions
Andersonam (talk | contribs) |
Andersonam (talk | contribs) |
||
| Line 19: | Line 19: | ||
==<i>Streptococcus pneumoniae</i>== | ==<i>Streptococcus pneumoniae</i>== | ||
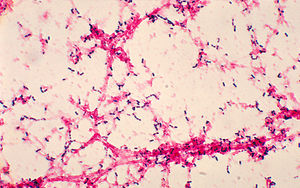
<i>Streptococcus pneumoniae</i>, also referred to as pneumococcus, is a gram-positive bacteria that belongs to the Firmicutes phylum. Figure 2 shows gram-stained <i>S. pneumoniae</i> grown on blood agar which is the frequent medium used in laboratories when researching this microorganism. It is a non-spore forming and non-motile bacteria [32]. <i>Streptococcus pneumoniae</i> most frequently are diplococci in structure, meaning they prefer to associate in pairs, but they can also be found individually or in short chains. Each individual cell measures between 0.5 and 1.25 micrometers in diameter [33]. Its optimal growth environment is 35-37 degrees Celsius [10]. This microorganism contains a circular genome with over 2 million base pairs [35]. Different strains of the bacteria can vary up to 10% in genetic information, but are consistent with a reoccurring set of 1553 genes [35]. Pneumococci are facultative anaerobes having the flexibility to ferment glucose into lactic acid for energy or utilize aerobic respiration for energy depending on the amount of oxygen available in its environment [32]. <i>S. pneumoniae</i> has the ability to oxidize hemoglobin characterizing it as an alpha-hemolytic bacteria. Its alpha-hemolytic property lends itself to laboratory identification of the microorganism since it can induce hemolysis on blood agar and, thus, appears green [33]. | |||
<br> | <br> | ||
[[Image:phil-2896.jpg|thumb|300px|left|Figure 2. Photomicrograph of gram-positive <i>Streptococcus pneumoniae</i> grown from blood culture, on 1978. By Dr. Mike Miller, at the [http://www.cdc.gov/ CDC].]] | [[Image:phil-2896.jpg|thumb|300px|left|Figure 2. Photomicrograph of gram-positive <i>Streptococcus pneumoniae</i> grown from blood culture, on 1978. By Dr. Mike Miller, at the [http://www.cdc.gov/ CDC].]] | ||
<br> | <br><i>Streptococcus pneumoniae</i> can live naturally in the human nasopharynx without causing disease. However, commensal carriage rates directly affect the transmission of <i>S. pneumoniae</i> [31] within any given population. Meningitis is sometimes said to be community-acquired because the carriage rates of <i>S. pneumoniae</i> increase in crowded situations. For example, children, who typically have the highest carriage rates nearing 37%, in crowded environments like that of day care centers have carriage rates that can reach as high as 58% [27]. Likewise, 50-60% of adult service personnel on military bases are likely to be carriers compared to the 5-10% of all healthy adults without children [11]. | ||
<br> | <br> | ||
<br> | <br>Pathogenic <i>S. pneumoniae</i> are encapsulated in polysaccharides whereas commensal <i>S. pneumoniae</i> do not have the same capsule. This capsule proves to be important to the virulence of the microorganism [11] in evading the human immune responses. When pathogenic, <i>S. pneumoniae</i> is responsible for pneumococcal disease, which includes meningitis, pneumonia, parapneumonic empyema, occult bacteremia, sepsis, arthritis, peritonitis, and endophthalmitis [3]. Transmission of <i>S. pneumoniae</i> most often occurs through respiratory droplets, in other words coughing or sneezing [11], making the means of contracting pneumococcal disease dangerously easy. | ||
<br> | <br> | ||
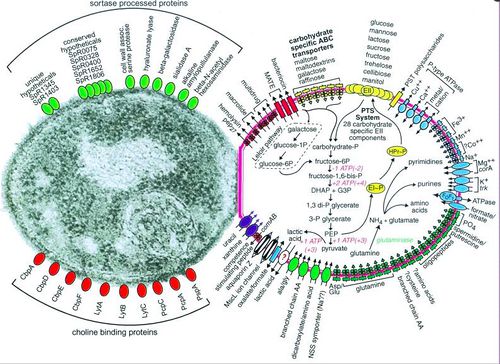
[[Image:surface-proteins.jpg|thumb|500px|right|Figure 3. Depiction of <i>Streptococcus pneumoniae</i> substrate transport, carbohydrate and glutamine metabolism, and selected categories of cell surface proteins, for full caption see paper Hoskins, et. al 2001 [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC95463/figure/F1/ PubMed].]] | [[Image:surface-proteins.jpg|thumb|500px|right|Figure 3. Depiction of <i>Streptococcus pneumoniae</i> substrate transport, carbohydrate and glutamine metabolism, and selected categories of cell surface proteins, for full caption see paper Hoskins, et. al 2001 [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC95463/figure/F1/ PubMed].]] | ||
<br> | <br> | ||
<br> | <br>Pneumococci has over 93 known serotypes that are differentiated based on the composition of capsular polysaccharide [9]. Only a few of these serotypes account for the majority of disease causing agents worldwide including serotypes 1, 5, 6A, 6B, 14, 19F, and 23F [20]. The serotype responsible for pneumococcal meningitis outbreaks in the African meningitis belt is serotype 1 [20]. | ||
==Pneumococcal Meningitis Pathogenesis== | ==Pneumococcal Meningitis Pathogenesis== | ||
Revision as of 12:36, 21 April 2015
Introduction
By Avery Anderson
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki. The insertion code consists of:
Double brackets: [[
Filename: PHIL_1181_lores.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
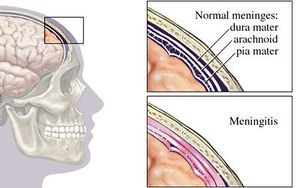
Bacterial meningitis is a potentially life-threatening disease of the central nervous system (CNS). It is characterized by inflammation of the membranes surrounding the brain and spinal cord, known as the meninges. The meninges include the pia mater, the arachnoid and the dura mater that encase and protect the brain and spinal cord. Figure 1 demonstrates the location of the meninges and shows a visual representation of inflammation that occurs during meningitis. The two leading causes of bacterial meningitis are Streptococcus pneumonia and Neisseria meningitidis [27]. Bacterial meningitis caused by Neisseria meningitidis is known as meningococcal meningitis while bacterial meningitis caused by Streptococcus pneumoniae is referred to as pneumococcal meningitis. Streptococcus pneumoniae accounts for two-thirds of all meningitis cases in the United States and Western Europe [1] and has the highest mortality rate of all other microorganisms causing meningitis [26]. In regions of the world where resources are scarce, fatality rates of pneumococcal meningitis near 51% and even in developed countries the rates are as high as 37% [7].
Streptococcus pneumoniae
Streptococcus pneumoniae, also referred to as pneumococcus, is a gram-positive bacteria that belongs to the Firmicutes phylum. Figure 2 shows gram-stained S. pneumoniae grown on blood agar which is the frequent medium used in laboratories when researching this microorganism. It is a non-spore forming and non-motile bacteria [32]. Streptococcus pneumoniae most frequently are diplococci in structure, meaning they prefer to associate in pairs, but they can also be found individually or in short chains. Each individual cell measures between 0.5 and 1.25 micrometers in diameter [33]. Its optimal growth environment is 35-37 degrees Celsius [10]. This microorganism contains a circular genome with over 2 million base pairs [35]. Different strains of the bacteria can vary up to 10% in genetic information, but are consistent with a reoccurring set of 1553 genes [35]. Pneumococci are facultative anaerobes having the flexibility to ferment glucose into lactic acid for energy or utilize aerobic respiration for energy depending on the amount of oxygen available in its environment [32]. S. pneumoniae has the ability to oxidize hemoglobin characterizing it as an alpha-hemolytic bacteria. Its alpha-hemolytic property lends itself to laboratory identification of the microorganism since it can induce hemolysis on blood agar and, thus, appears green [33].
Streptococcus pneumoniae can live naturally in the human nasopharynx without causing disease. However, commensal carriage rates directly affect the transmission of S. pneumoniae [31] within any given population. Meningitis is sometimes said to be community-acquired because the carriage rates of S. pneumoniae increase in crowded situations. For example, children, who typically have the highest carriage rates nearing 37%, in crowded environments like that of day care centers have carriage rates that can reach as high as 58% [27]. Likewise, 50-60% of adult service personnel on military bases are likely to be carriers compared to the 5-10% of all healthy adults without children [11].
Pathogenic S. pneumoniae are encapsulated in polysaccharides whereas commensal S. pneumoniae do not have the same capsule. This capsule proves to be important to the virulence of the microorganism [11] in evading the human immune responses. When pathogenic, S. pneumoniae is responsible for pneumococcal disease, which includes meningitis, pneumonia, parapneumonic empyema, occult bacteremia, sepsis, arthritis, peritonitis, and endophthalmitis [3]. Transmission of S. pneumoniae most often occurs through respiratory droplets, in other words coughing or sneezing [11], making the means of contracting pneumococcal disease dangerously easy.
Pneumococci has over 93 known serotypes that are differentiated based on the composition of capsular polysaccharide [9]. Only a few of these serotypes account for the majority of disease causing agents worldwide including serotypes 1, 5, 6A, 6B, 14, 19F, and 23F [20]. The serotype responsible for pneumococcal meningitis outbreaks in the African meningitis belt is serotype 1 [20].
Pneumococcal Meningitis Pathogenesis
Before Streptococcus pneumoniae has the chance to ruminate as pneumococcal meningitis in the brain and spinal cord it must first find a way to colonize in the nasopharynx mucus, invade the mucosal epithelium, survive in the bloodstream, and traverse the blood-brain barrier [21]. Streptococcus pneumoniae are estimated to possess over 500 surface proteins that allow for interaction between the microorganism and the host [21]. The genes encoding these surface proteins are known as virulence genes because they are largely responsible for the microorganisms ability to infect and cause disease in the host [32]. Of these surface proteins, choline-binding proteins are especially important in agitating the inflammatory response in the brain that is characteristic of meningitis. Figure 3 depicts the many surface proteins on pneumococci. One of the first human defenses against S. pneumoniae involves respiratory mucus that essentially block the bacteria from adhering to the epithelial cell layer and lysozymes which are enzymes that catalyze the break down of the bacterial cell wall [29]. S. pneumoniae is able to evade these defenses by its expression of enzymes (NanA, BgaA, StrH, and NanB) that decrease mucus viscosity, thus increasing the difficulty for the human defense mucus layer to entrap the bacteria. Additionally, the negative charge of the pneumococci capsule allows it to repel from sialic acid of the mucus further evading entrapment [27]. To escape destruction by lysozymes, S. pneumoniae expresses two enzymes, peptidoglycan N-acetylglucosamine-deacetylase A (PdgA) and O-acetyltransferase (Adr), that modify the peptidoglycan consequently inhibiting lysozyme cleavage [14].
Immunoglobulin A (IgA), an antibody released by the human body for immunity, is unable to bind to the S. pneumoniae because of the polysaccharide capsule and the pathogen is able to cleave the IgA with IgA protease [27]. One of the surface proteins on the pneumococci, surface protein A (PspA), binds lactoferrin, a immune system protein with antimicrobial properties, preventing its bactericidal activity. Pneumococci have the advantageous ability to manipulate the binding properties of its capsule through phase variation, adjusting the polysaccharide composition of the capsule [27]. To evade the initial immune defenses, the pneumococcal capsule is thick, opaque, to protect it against entrapment by mucus or breakdown from immunoglobulin and complement binding [18]. Following survival of those mechanisms, the capsule is thin, transparent, exposing adhesion molecules to interact with the host nasopharyngeal epithelium, the necessary step to gain entry to the bloodstream [36]. The microorganism utilizes its surface proteins, namely the phosphorylcholine to bind to the platelet activating factor (PAF) receptor and choline-binding protein PspC, and binds to glycoconjugates on the epithelium to facilitate cell transcytosis, crossing through the epithelium cells into the bloodstream [27].
Surviving immune defenses in the bloodstream requires the protection of the polysaccharide capsule and binding of surface proteins to complement components. Pneumolysin is a virulence factor released by S. pneumoniae that allows the microorangism to escape phagocytosis by decreasing complement factors [2].
Similarly to the transcytosis process of the epithelium cells, S. pneumoniae in the transparent capsule variation binds phosphoylcholine to PAF receptors of endothelial cells. Pneumococcal surface protein C (Psp C) additionally binds to laminin receptors on the endothelial cell facilitating S. pneumoniae entry into cerebral spinal fluid [19]. Disruption of tight junctions between endothelial cells also appears to be necessary for the disease to translocate across the blood-brain barrier [19]. When the pneumococci reach the cerebral spinal fluid they are able to multiply freely. Pneumococci binding to pattern recognition receptors sets off a cascade of inflammatory reactions initiated by the release of cytokines from antigen-presenting cells (APCs) [27]. This process involves the recruitment of white blood cells, including neutrophils and lymphocytes, from the blood to the region of S. pneumoniae occupation [27]. The APCs are present in various locations in the brain including the cerebral spinal fluid, meninges, choroid plexus, or perivascular space [13]. Stimulating the pattern recognition receptors of ACPs up regulates the production of cytokines, expressing a pro-inflammatory response [27]. Leukocytes are attracted into the cerebral spinal fluid by pro-inflammatory cytokines as well as cerebral perivascular and meningeal macrophages [27]. The following increase in release of reactive oxygen species and reactive nitrogen species leads to destabilizing oxidative stress in the brain [15].
Interaction with other microbial species
Include some current research, with at least one figure showing data.
Symptoms & Diagnosis
Include some current research, with at least one figure showing data.
Risk Factors
Neurological sequelae
Treatment & Adjunctive Therapies
Conclusion
References
[1] Hodgkin, J. and Partridge, F.A. "Caenorhabditis elegans meets microsporidia: the nematode killers from Paris." 2008. PLoS Biology 6:2634-2637.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2015, Kenyon College.
