Xenorhabdus nematophilus: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
In the introduction, give a brief overview of the microbial interaction that is the topic of this page. Introduce the interaction, the organisms involved, the ecological significance of this interaction, and the importance of microorganisms and their processes in this environment (described in more detail below). What processes do they carry out? What functions do they perform? Why are microbes important in this interaction? | |||
==Classification== | ==Classification== | ||
Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacteriales; Enterobacteriaceae;Xenorhabdus | Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacteriales; Enterobacteriaceae;Xenorhabdus | ||
| Line 14: | Line 15: | ||
<i>Xenorhabdus nematophilus</i> is not found free living in the soil environment. It exists in a symbiotic relationship with insect-parasitizing nematodes of <i>Steinernema carpocapsae </i>. The interaction is specific to each species. They are found ubiquitously in soil environments. Their ecological significance is particularly apparent in agriculture, as a form of biological control of pest insect species. The biological processes of the bacterium are matched by the needs of the nematode and vice versa. Together this mutualistic relationship results in the predation of insect species, such as those in the order Lepidoptera. | <i>Xenorhabdus nematophilus</i> is not found free living in the soil environment. It exists in a symbiotic relationship with insect-parasitizing nematodes of <i>Steinernema carpocapsae </i>. The interaction is specific to each species. They are found ubiquitously in soil environments. Their ecological significance is particularly apparent in agriculture, as a form of biological control of pest insect species. The biological processes of the bacterium are matched by the needs of the nematode and vice versa. Together this mutualistic relationship results in the predation of insect species, such as those in the order Lepidoptera. | ||
===Morphology=== | ===Important Morphology=== | ||
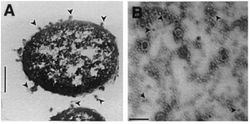
Gram-negative bacteria produce two-layer outer membrane blebs. As outer membrane vesicles, these spherical structures encapsulate toxic proteins to protect them from the degradation by host enzymes | Gram-negative bacteria produce two-layer outer membrane blebs. As outer membrane vesicles, these spherical structures encapsulate toxic proteins to protect them from the degradation by host enzymes. <i>X. nematophilus</i> excrete blebs into its growth medium [SOURCE:khandelwal]. The vesicles have electron-dense contents and also contained proteins. The excretions have properties of insecticides, antimicrobial agents, and degradative enzyms. [KHAND, SOURCES] | ||
===Life Cycle=== | ===Life Cycle=== | ||
| Line 27: | Line 28: | ||
[[Image:Shapiro.cornell.process.jpg|thumb|300px|left|The life cycle of the nematode is completed in a few days, and it results in thousands of new infective juveniles looking for hosts. Source: http://www.biocontrol.entomology.cornell.edu/pathogens/nematodes.html]] | [[Image:Shapiro.cornell.process.jpg|thumb|300px|left|The life cycle of the nematode is completed in a few days, and it results in thousands of new infective juveniles looking for hosts. Source: http://www.biocontrol.entomology.cornell.edu/pathogens/nematodes.html]] | ||
The interaction between <i>Xenorhabdus nematophilus</i> and Steinernema carpocapsae is a specific mutualism. The bacterium is contained in a vesicle of the | The interaction between <i>Xenorhabdus nematophilus</i> and Steinernema carpocapsae is a specific mutualism. The bacterium is contained in a vesicle of the foregut of an infective juvenile stage soil nematode. Once the nematode finds and enters an insect host, the bacterium in released into the hemocoel of the insect. | ||
===Why <i>S. carpocapsae</i> needs <i>X. nematophilus</i> | ===Why <i>S. carpocapsae</i> needs <i>X. nematophilus</i>=== | ||
The nematode is not a free living organism and requires a host for its life cycle to be completed. Only the infective juvenile stage of <i>S. carpocapsae</i> is able to move through the environment in order to find a new host. | The nematode is not a free living organism and requires a host for its life cycle to be completed. Only the infective juvenile stage of <i>S. carpocapsae</i> is able to move through the environment in order to find a new host. In order to create an optimal growing environment in which to grow and reproduce, the nematode needs the entomopathogenic bacterial symbiont. <i>X. nematophilus</i> will secrete compounds that kill the insect host (found to be toxic proteins). Additional compounds include enzymes that bioconvert the insect host. Antimicrobial agents are also secreted that protect the insect carcass from putrification from other microbes. [Khandelwal, Floyd]. In the initial stages of infection of the insect host, <i>X. nematophilus</i> inhibits the growth of various fungal and bacterial competitors. The metabolites exuded by the bacterium are known to have antifungal, nematicidal, or insecticidal effects. <i>X. nematophilus</i> limits competition so that the nematode and itself can use the insect larvae carcass nutrients. | ||
In the initial stages of infection of the insect host, <i>X. nematophilus</i> inhibits the growth of various fungal and bacterial competitors. The metabolites exuded by the bacterium are known to have antifungal, nematicidal, or insecticidal effects. | |||
<i>X. nematophilus</i> | |||
===Why <i>X. nematophilus</i> needs <i>S. carpocapsae</i>=== | |||
The bacterium <i>X. nematophilus</i> is not found in the soil environment. It cannot survive in water or soil for long by itself, as it is an endosymbiont of nematodes (<i>Photorhabdus luminescens</i> has the same characteristic). | |||
<i>S. carpocapsae</i> facilitates the death of the larval host. The nematode is only free living during its infective juvenile stage, and it is at this time it can disperse through the soil environment to locate a larval host. This function has allowed both organisms to become ubiquitous in nearly all soil environments. | |||
The nematode | |||
When a host is found, <i>S. carpocapsae</i> enters the body cavity of the larvae. Once it is in the hemocoel of the insect, the <i>X. nematophila</i> are released from the gut of the nematode.The hemocoel is a series of interconnected tissue spaces through which blood circulates in arthropods, thus it is an ideal structure to start an infection. | |||
===How interaction influences community | |||
===How interaction influences populations=== | |||
LD50: the dose of a chemical that causes the death of 50% of a test population. For <i>Helicoverpa armigera</i> (corn earworm) the LD50= 4x10<sup>4</sup> cells/gram of diet. Besides non-fatal levels of infection resulted in symptoms such as small size and moribund (near death). These results are true for when the bacterium invades the homocoel of the larva or is ingested orally. [SOURCE KHANDEL] | |||
===How interaction influences community=== | |||
In Agricultural systems. The use of <i>X. nematophilus</i> as a bio-pesticide is limited. It cannot be stored indefinitely on a shelf under any conditions. For the bacterium to be effective, it needs to be transported and used within a time frame. And as shown by the OMV research, it cannot be exposed to excessive heat. This can be a topic of further research. LINK. | In Agricultural systems. The use of <i>X. nematophilus</i> as a bio-pesticide is limited. It cannot be stored indefinitely on a shelf under any conditions. For the bacterium to be effective, it needs to be transported and used within a time frame. And as shown by the OMV research, it cannot be exposed to excessive heat. This can be a topic of further research. LINK. | ||
| Line 64: | Line 59: | ||
<i>X. nematophilus</i> has antagonistic interactions with entomopathogenic fungi. | <i>X. nematophilus</i> has antagonistic interactions with entomopathogenic fungi. | ||
==Niche== | ==Niche== | ||
<i>Xenorhabdus nematophilus</i> is ubiquitous across all habitat types. Since it has been shown that Steinernematid (and Heterorhabditid) nematodes are exclusive to soil environments, and they have been isolated from every continent, excluding Antarctica. Entomopathogenic nematodes exist in a diverse range of soil habitats including farmland, forests, beaches, and deserts. A survey of entomopathogenic nematodes had confirmation of the nematodes in 2-25% of the sites sampled. | <i>Xenorhabdus nematophilus</i> is ubiquitous across all habitat types. Since it has been shown that Steinernematid (and Heterorhabditid) nematodes are exclusive to soil environments, and they have been isolated from every continent, excluding Antarctica. Entomopathogenic nematodes exist in a diverse range of soil habitats including farmland, forests, beaches, and deserts. A survey of entomopathogenic nematodes had confirmation of the nematodes in 2-25% of the sites sampled. | ||
| Line 73: | Line 68: | ||
[[Image:Shapiro.cornell.jpg|thumb|200px|right| <b>A</b> Entomopathogenic nematode, infective juevenile stage. <b>B</b> Infected Galleria mellonella (wax moth) larva with nematodes emerging. Source: http://www.biocontrol.entomology.cornell.edu/pathogens/nematodes.html]] | [[Image:Shapiro.cornell.jpg|thumb|200px|right| <b>A</b> Entomopathogenic nematode, infective juevenile stage. <b>B</b> Infected Galleria mellonella (wax moth) larva with nematodes emerging. Source: http://www.biocontrol.entomology.cornell.edu/pathogens/nematodes.html]] | ||
Helicoverpa armigera | |||
The cotton bollworm, corn earworm or Old World bollworm, Helicoverpa armigera, is a moth, the larvae of which feed on a wide range of plants, including many important cultivated crops. Wikipedia | |||
Scientific name: Helicoverpa armigera | |||
===Phases of <i>X. nematophilus</i> 1=== | ===Phases of <i>X. nematophilus</i> 1=== | ||
<i>X. nematophilus</i> is believed to have phase I and phase II forms. Xn phase one motile. peritrichous flagella. Xn phase two non motile. but variable depending on environmental conditions. | <i>X. nematophilus</i> is believed to have phase I and phase II forms. Xn phase one motile. peritrichous flagella. Xn phase two non motile. but variable depending on environmental conditions. | ||
====Phase I | ====Phase I==== | ||
====Phase II | ====Phase II==== | ||
The outer membrane OM of gram-negative bacteria performs many functions. They may be specialized to increase survival in adverse conditions. The OM has a mechanism for protein secretion, that in the case of bacterial pathogens will "inactivate" the host. establish. | The outer membrane OM of gram-negative bacteria performs many functions. They may be specialized to increase survival in adverse conditions. The OM has a mechanism for protein secretion, that in the case of bacterial pathogens will "inactivate" the host. establish. | ||
| Line 104: | Line 103: | ||
==Microbial processes== | ==Microbial processes== | ||
===Bacterium Functions=== | |||
===Bacterium Functions | |||
====Toxic proteins==== | ====Toxic proteins==== | ||
morgan et al. there is a DNA region of Xn found to encode for insecticidal protein, chitinase, and other pathogenic attributes. This suggests that the toxins of Xn are made up of multiple components. (multiple polypeptides) | morgan et al. there is a DNA region of Xn found to encode for insecticidal protein, chitinase, and other pathogenic attributes. This suggests that the toxins of Xn are made up of multiple components. (multiple polypeptides) | ||
TOXINS: the particulate fraction of the insecticidal proteins are more active than the soluable fraction (high-speed centrifugation). "Heating OMVs at 80 C for 15 min inactivated the insecticidal activity"When treated with a proteinase, up to 80% of the OMV proteins were degraded and inactive. | TOXINS: the particulate fraction of the insecticidal proteins are more active than the soluable fraction (high-speed centrifugation). "Heating OMVs at 80 C for 15 min inactivated the insecticidal activity"When treated with a proteinase, up to 80% of the OMV proteins were degraded and inactive. | ||
====Outer Membrane Vesicles | ====Outer Membrane Vesicles==== | ||
OMV emanate from the cell, encapsulate toxic proteins- protects proteins from degradation by the cells enzymes. vesicles are like a delivery system for the cell. separated from the cytosol and in most organisms is separated by at least one lipid bilayer. During growth, OMV are sloughed off from the surface of a cell. OMVs for Sn contain electron-dense compounds, proteins, porins, | OMV emanate from the cell, encapsulate toxic proteins- protects proteins from degradation by the cells enzymes. vesicles are like a delivery system for the cell. separated from the cytosol and in most organisms is separated by at least one lipid bilayer. During growth, OMV are sloughed off from the surface of a cell. OMVs for Sn contain electron-dense compounds, proteins, porins, | ||
Abiosis excretions | Abiosis excretions | ||
| Line 116: | Line 114: | ||
Nutrient excretions | Nutrient excretions | ||
===Nematode Functions | ===Nematode Functions=== | ||
Nematodes are classified as part of the microfauna in a soil environment.Food web studies show that fauna contribute up to 30% of the total net nitrogen mineralization, mainly from microbial-feeding nematodes and protozoa. At this level of the soil ecosystem, nitrogen and other nutrients that would otherwise be locked up in organic form, are released through the decomposition activities of the microfauna. The possible Net Primary Productivity of the system is increased. Specific to the interaction between <i>X. nematophilus</i> and <i>S. carpocapsae</i>, the insect carcass is consumed by the nematode and the bacterium, efficiently using all available nutrients. The symbiotic relationship between the two organisms allows for maximum uptake or release of nutrients. | Nematodes are classified as part of the microfauna in a soil environment.Food web studies show that fauna contribute up to 30% of the total net nitrogen mineralization, mainly from microbial-feeding nematodes and protozoa. At this level of the soil ecosystem, nitrogen and other nutrients that would otherwise be locked up in organic form, are released through the decomposition activities of the microfauna. The possible Net Primary Productivity of the system is increased. Specific to the interaction between <i>X. nematophilus</i> and <i>S. carpocapsae</i>, the insect carcass is consumed by the nematode and the bacterium, efficiently using all available nutrients. The symbiotic relationship between the two organisms allows for maximum uptake or release of nutrients. | ||
Termite have microbial symbionts in the [[termite gut]], and together the two organisms decompose ligno-cellulose. | Termite have microbial symbionts in the [[termite gut]], and together the two organisms decompose ligno-cellulose. | ||
| Line 144: | Line 142: | ||
2. Codispoti, L.A. et al. "High Nitrite Levels off Northern Peru: A Signal of Instability in the Marine Denitrification Rate". Science. 1986. Volume 233. p.1200-1202. | 2. Codispoti, L.A. et al. "High Nitrite Levels off Northern Peru: A Signal of Instability in the Marine Denitrification Rate". Science. 1986. Volume 233. p.1200-1202. | ||
3. Schulz, H., Jorgensen, B., Fossing, H., and Ramsing, N. "Community Structure of Filamentous, Sheath-Building Sulfur Bacteria, Thioploca spp., off the Coast of Chile". Applied and Environmental Microbiology. 1996. Volume 62. p. 1855-1862. | 3. Schulz, H., Jorgensen, B., Fossing, H., and Ramsing, N. "Community Structure of Filamentous, Sheath-Building Sulfur Bacteria, Thioploca spp., off the Coast of Chile". Applied and Environmental Microbiology. 1996. Volume 62. p. 1855-1862. | ||
10. Teske, A., Ramsing, N. B., Kuver J., and Fossing, H. "Phylogeny of Thioploca and related filamentous sulfide-oxidizing bacteria". Syst. Appl. Microbiology. 1996. Volume 18. p. 517-526. | 10. Teske, A., Ramsing, N. B., Kuver J., and Fossing, H. "Phylogeny of Thioploca and related filamentous sulfide-oxidizing bacteria". Syst. Appl. Microbiology. 1996. Volume 18. p. 517-526. | ||
11. Larking, J., and Strohl, W. "Beggiatoa, Thiothrix, and Thioploca". Ann. Rev. Microbiology. 1983. Volume 37. p. 361-362. | 11. Larking, J., and Strohl, W. "Beggiatoa, Thiothrix, and Thioploca". Ann. Rev. Microbiology. 1983. Volume 37. p. 361-362. | ||
Revision as of 02:36, 9 April 2013
In the introduction, give a brief overview of the microbial interaction that is the topic of this page. Introduce the interaction, the organisms involved, the ecological significance of this interaction, and the importance of microorganisms and their processes in this environment (described in more detail below). What processes do they carry out? What functions do they perform? Why are microbes important in this interaction?
Classification
Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacteriales; Enterobacteriaceae;Xenorhabdus
Species
Xenorhabdus nematophilus Taxonomy
Introduction
Xenorhabdus nematophilus is a gram-negative bacterium in the family Enterobacteriaceae. This microbe can be described as entomopathogenic (i.e. an organism which can kill arthropods by poisoning with its own toxins or those it harbors).
Xenorhabdus nematophilus is not found free living in the soil environment. It exists in a symbiotic relationship with insect-parasitizing nematodes of Steinernema carpocapsae . The interaction is specific to each species. They are found ubiquitously in soil environments. Their ecological significance is particularly apparent in agriculture, as a form of biological control of pest insect species. The biological processes of the bacterium are matched by the needs of the nematode and vice versa. Together this mutualistic relationship results in the predation of insect species, such as those in the order Lepidoptera.
Important Morphology
Gram-negative bacteria produce two-layer outer membrane blebs. As outer membrane vesicles, these spherical structures encapsulate toxic proteins to protect them from the degradation by host enzymes. X. nematophilus excrete blebs into its growth medium [SOURCE:khandelwal]. The vesicles have electron-dense contents and also contained proteins. The excretions have properties of insecticides, antimicrobial agents, and degradative enzyms. [KHAND, SOURCES]
Life Cycle
The life cycle of this microbe has been described as having two phases, where it will secrete different metabolites.
Xenorhabdus nematophilus is a bacterium
Biological interaction
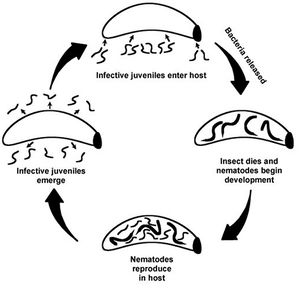
The interaction between Xenorhabdus nematophilus and Steinernema carpocapsae is a specific mutualism. The bacterium is contained in a vesicle of the foregut of an infective juvenile stage soil nematode. Once the nematode finds and enters an insect host, the bacterium in released into the hemocoel of the insect.
Why S. carpocapsae needs X. nematophilus
The nematode is not a free living organism and requires a host for its life cycle to be completed. Only the infective juvenile stage of S. carpocapsae is able to move through the environment in order to find a new host. In order to create an optimal growing environment in which to grow and reproduce, the nematode needs the entomopathogenic bacterial symbiont. X. nematophilus will secrete compounds that kill the insect host (found to be toxic proteins). Additional compounds include enzymes that bioconvert the insect host. Antimicrobial agents are also secreted that protect the insect carcass from putrification from other microbes. [Khandelwal, Floyd]. In the initial stages of infection of the insect host, X. nematophilus inhibits the growth of various fungal and bacterial competitors. The metabolites exuded by the bacterium are known to have antifungal, nematicidal, or insecticidal effects. X. nematophilus limits competition so that the nematode and itself can use the insect larvae carcass nutrients.
Why X. nematophilus needs S. carpocapsae
The bacterium X. nematophilus is not found in the soil environment. It cannot survive in water or soil for long by itself, as it is an endosymbiont of nematodes (Photorhabdus luminescens has the same characteristic).
S. carpocapsae facilitates the death of the larval host. The nematode is only free living during its infective juvenile stage, and it is at this time it can disperse through the soil environment to locate a larval host. This function has allowed both organisms to become ubiquitous in nearly all soil environments.
When a host is found, S. carpocapsae enters the body cavity of the larvae. Once it is in the hemocoel of the insect, the X. nematophila are released from the gut of the nematode.The hemocoel is a series of interconnected tissue spaces through which blood circulates in arthropods, thus it is an ideal structure to start an infection.
How interaction influences populations
LD50: the dose of a chemical that causes the death of 50% of a test population. For Helicoverpa armigera (corn earworm) the LD50= 4x104 cells/gram of diet. Besides non-fatal levels of infection resulted in symptoms such as small size and moribund (near death). These results are true for when the bacterium invades the homocoel of the larva or is ingested orally. [SOURCE KHANDEL]
How interaction influences community
In Agricultural systems. The use of X. nematophilus as a bio-pesticide is limited. It cannot be stored indefinitely on a shelf under any conditions. For the bacterium to be effective, it needs to be transported and used within a time frame. And as shown by the OMV research, it cannot be exposed to excessive heat. This can be a topic of further research. LINK.
Interactions with other species 5
potato
Phtytophthora infestants oomycete negative/positive?. outcome? “”However, earlier identified soluble antibiotics, such as xenorhabdins, indole compounds (McInerey et al., 1991a, b) and nematophin (Li et al., 1997) from other Xenorhabdus spp. have shown antifungal activity against soil fungi.""
fungi
X. nematophilus has antagonistic interactions with entomopathogenic fungi.
Niche
Xenorhabdus nematophilus is ubiquitous across all habitat types. Since it has been shown that Steinernematid (and Heterorhabditid) nematodes are exclusive to soil environments, and they have been isolated from every continent, excluding Antarctica. Entomopathogenic nematodes exist in a diverse range of soil habitats including farmland, forests, beaches, and deserts. A survey of entomopathogenic nematodes had confirmation of the nematodes in 2-25% of the sites sampled.
Cultivated Fields1
Subsection 2
Subsection 2
Key Microorganisms, Microbial Communities
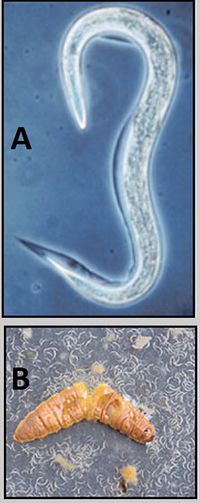
Helicoverpa armigera
The cotton bollworm, corn earworm or Old World bollworm, Helicoverpa armigera, is a moth, the larvae of which feed on a wide range of plants, including many important cultivated crops. Wikipedia Scientific name: Helicoverpa armigera
Phases of X. nematophilus 1
X. nematophilus is believed to have phase I and phase II forms. Xn phase one motile. peritrichous flagella. Xn phase two non motile. but variable depending on environmental conditions.
Phase I
Phase II
The outer membrane OM of gram-negative bacteria performs many functions. They may be specialized to increase survival in adverse conditions. The OM has a mechanism for protein secretion, that in the case of bacterial pathogens will "inactivate" the host. establish. virulence factors are contained in OMV and excreted. Pseudomonas aeruginosa, Proteus mirabilis, and Serratia marcescens ---package enzymes (proelastases, hemolysins, protease. BACT. Bacteroides gingivalis has similar visicle properties. (toxicity, release toxins from OMV)
Other Microbes
photorhabdus (luminescens Xn related to photorhabdus (luminescens (homology of genese coding for the insecticidal proteins,associated with soil nematodes) Photorhabdus luminescens also has oral insecticidal activity
Nematode functions 2
Insect function 3
Species in the order Lepidoptera have numerous roles in an ecosystem. HOSTS: Lepidoptera. pest: Helicoverpa armigera. Pieris brassicae (cabbage white butterfly) larvae Manduca sexta. greater wax moth, Galleria mellonella.
Adult
Pollination, Dispersal Food source (bats, small mammals, carnivorious insects)
=Larval
Crop Damage, Agriculture (food source) Forest, tree damage (wood borers) - coleoptera though
other
potato/fungi Phtytophthora infestants
Microbial processes
Bacterium Functions
Toxic proteins
morgan et al. there is a DNA region of Xn found to encode for insecticidal protein, chitinase, and other pathogenic attributes. This suggests that the toxins of Xn are made up of multiple components. (multiple polypeptides)
TOXINS: the particulate fraction of the insecticidal proteins are more active than the soluable fraction (high-speed centrifugation). "Heating OMVs at 80 C for 15 min inactivated the insecticidal activity"When treated with a proteinase, up to 80% of the OMV proteins were degraded and inactive.
Outer Membrane Vesicles
OMV emanate from the cell, encapsulate toxic proteins- protects proteins from degradation by the cells enzymes. vesicles are like a delivery system for the cell. separated from the cytosol and in most organisms is separated by at least one lipid bilayer. During growth, OMV are sloughed off from the surface of a cell. OMVs for Sn contain electron-dense compounds, proteins, porins, Abiosis excretions the bacteria produce antibiosis in vivo (in an organism) and in vitro (in artificial) Nutrient excretions
Nematode Functions
Nematodes are classified as part of the microfauna in a soil environment.Food web studies show that fauna contribute up to 30% of the total net nitrogen mineralization, mainly from microbial-feeding nematodes and protozoa. At this level of the soil ecosystem, nitrogen and other nutrients that would otherwise be locked up in organic form, are released through the decomposition activities of the microfauna. The possible Net Primary Productivity of the system is increased. Specific to the interaction between X. nematophilus and S. carpocapsae, the insect carcass is consumed by the nematode and the bacterium, efficiently using all available nutrients. The symbiotic relationship between the two organisms allows for maximum uptake or release of nutrients. Termite have microbial symbionts in the termite gut, and together the two organisms decompose ligno-cellulose.
Current Research
Biological Control
Agriculture. the most successful insecticide based on a microbe is that from the bacterium Bacillus thuringiensis. issue: resistence. so look for new genes. The genes must be orally active protein toxins. Such that the gene can be genetically engineered to be expressed in the plant tissue eaten by the pests. This is important because then only the insect species that are damaging the crops will be killed. This allows for safer pesticide use in cultivated fields, and it allows for greater biodiversity.
What factors allow for the bacteria to recognize environment?
The bacteria must have some mechanism by which to recognize whether it is in it’s host environment and regulate gene expression accordingly. ===What are the specific functions of the proteins in the outer membrane vesicles? unable to determine the toxicity individual proteins present in OMV mixtures for Xn. They confirmed that the OMV contain insecticidal protein toxins. Chitinase is likely to be an OM protein, but its effectiveness is not known. The presence of chitinase increases the pathogenic possibilities of the OMV compounds for Xn.
References
1. Floyd L. Inman and Leonard Holmes, 2012. Effect of Heat Sterilization on the Bioactivity of Antibacterial Metabolites Secreted byXenorhabdus nematophila. Pakistan Journal of Biological Sciences, 15: 997-1000.
1. H. Fossing, V.A. Gallardo, et. al. "Concentration and transport of nitrate by the mat-forming sulphur bacterium Thioploca". Nature. 1995. Volume 374. p.713-715. 2. Codispoti, L.A. et al. "High Nitrite Levels off Northern Peru: A Signal of Instability in the Marine Denitrification Rate". Science. 1986. Volume 233. p.1200-1202. 3. Schulz, H., Jorgensen, B., Fossing, H., and Ramsing, N. "Community Structure of Filamentous, Sheath-Building Sulfur Bacteria, Thioploca spp., off the Coast of Chile". Applied and Environmental Microbiology. 1996. Volume 62. p. 1855-1862. 10. Teske, A., Ramsing, N. B., Kuver J., and Fossing, H. "Phylogeny of Thioploca and related filamentous sulfide-oxidizing bacteria". Syst. Appl. Microbiology. 1996. Volume 18. p. 517-526. 11. Larking, J., and Strohl, W. "Beggiatoa, Thiothrix, and Thioploca". Ann. Rev. Microbiology. 1983. Volume 37. p. 361-362. 12. Schulz, Heide, and Schulz, Horst "Large Sulfur Bacteria and the Formation of Phosphorite". Science. 2005. Volume 307. p. 416-418.
Edited by <Chloe M. Mattia>, a student of Angela Kent at the University of Illinois at Urbana-Champaign.