Xenorhabdus nematophilus
Classification
Bacteria; Proteobacteria; Gammaproteobacteria; Enterobacteriales; Enterobacteriaceae;Xenorhabdus
Species
Xenorhabdus nematophilus Taxonomy
Introduction
Xenorhabdus nematophilus is a gram-negative bacterium in the family Enterobacteriaceae. This microbe can be described as entomopathogenic (i.e. an organism which can kill arthropods by poisoning with its own toxins or those it harbors).
Xenorhabdus nematophilus is not found free living in the soil environment. It exists in a symbiotic relationship with insect-parasitizing nematodes of Steinernema carpocapsae . The interaction is specific to each species. They are found ubiquitously in soil environments. Their ecological significance is particularly apparent in agriculture, as a form of biological control of pest insect species. The biological processes of the bacterium are matched by the needs of the nematode and vice versa. Together this mutualistic relationship results in the predation of insect species, such as those in the order Lepidoptera.
Morphology
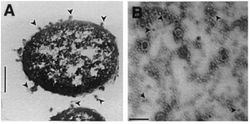
Gram-negative bacteria produce two-layer outer membrane blebs. As outer membrane vesicles, these spherical structures encapsulate toxic proteins to protect them from the degradation by host enzymes [khandelwal]. X. nematophilus uses the outer membrane vesicles as a way of excreting their insecticidal toxins. This microbe can be described as entomopathogenic (i.e. an organism which can kill arthropods by poisoning with its own toxins or those it harbors).
Life Cycle
The life cycle of this microbe has been described as having two phases, where it will secrete different metabolites.
Xenorhabdus nematophilus is a bacterium
Biological interaction
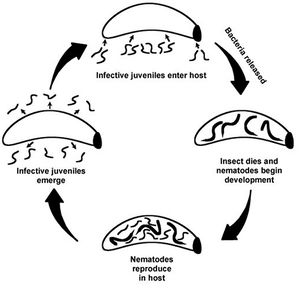
The interaction between Xenorhabdus nematophilus and Steinernema carpocapsae is a specific mutualism. The bacterium is contained in a vesicle of the intestine of an infective juveile stage nematode. Once the nematode finds and enters an insect host, the bacterium in released into the hemocoel of the insect.
Why S. carpocapsae needs X. nematophilus 1
The nematode is not a free living organism and requires a host for its life cycle to be completed. Only the infective juvenile stage of S. carpocapsae is able to move through the environment in order to find a new host.
Limited competition 1a
In the initial stages of infection of the insect host, X. nematophilus inhibits the growth of various fungal and bacterial competitors. The metabolites exuded by the bacterium are known to have antifungal, nematicidal, or insecticidal effects.
Effectively kills the host 1b
The Steinernema carpocapsae species of nematode is only free living during its juvenile stage. It is at this time that they are infective and seek out hosts. The nematode needs the bacterium to effectively kill the insect host and complete its life cycle. X. nematophilus excretes highly toxic and potent protein compounds that kills the host insect. The bacteria provide nutrients for the nematode development. The insect host dies and its body is a rich nutrient source for both the bacterium and the nematode. LD50: the dose of a chemical that causes the death of 50% of a test population. For H. armigera the LD50= 40000. Besides non-fatal levels of infection resulted in symptoms such as small size and moribund (near death). These results are true for when the bacterium invades the homocoel of the larva or is ingested orally.
Why X. nematophilus needs S. carpocapsae 2
The bacterium X. nematophilus is not found in the soil environment. It cannot survive in water or soil for long by itself; it needs the nematode (Photorhabdus has the same characteristic).
Finds Host 2a
finds, enters/penetrates dispersal
Effectively kills the host 2b
The nematode allows the bacterium to enter the homocoel of the insect. DEFINE homocoel: a series of interconnected open tissue spaces through which blood circulates, occurring in several invertebrate groups
endo symbionts in the foreguts of soil nematodes. When nematode is in the homocoel of the insect, the bacteria are released from the gut
How interaction influences Population 3
How interaction influences community 4
In Agricultural systems. The use of X. nematophilus as a bio-pesticide is limited. It cannot be stored indefinitely on a shelf under any conditions. For the bacterium to be effective, it needs to be transported and used within a time frame. And as shown by the OMV research, it cannot be exposed to excessive heat. This can be a topic of further research. LINK.
Interactions with other species 5
potato
Phtytophthora infestants oomycete negative/positive?. outcome? “”However, earlier identified soluble antibiotics, such as xenorhabdins, indole compounds (McInerey et al., 1991a, b) and nematophin (Li et al., 1997) from other Xenorhabdus spp. have shown antifungal activity against soil fungi.""
fungi
X. nematophilus has antagonistic interactions with entomopathogenic fungi.
<<Provide details of the symbiosis or biological interaction. Is this a specific or general interaction? How do these interactions influence the host or other microbial populations, and their activities? How do these interactions influence other organisms (positive or negative influences)? What is the outcome of this interaction? Are there ecological consequences? Describe biological interactions using as many sections/subsections as you require. Look at other topics available in MicrobeWiki. Create links where relevant.>>
Niche
Xenorhabdus nematophilus is ubiquitous across all habitat types. Since it has been shown that Steinernematid (and Heterorhabditid) nematodes are exclusive to soil environments, and they have been isolated from every continent, excluding Antarctica. Entomopathogenic nematodes exist in a diverse range of soil habitats including farmland, forests, beaches, and deserts. A survey of entomopathogenic nematodes had confirmation of the nematodes in 2-25% of the sites sampled.
Cultivated Fields1
Subsection 2
Subsection 2
Key Microorganisms, Microbial Communities
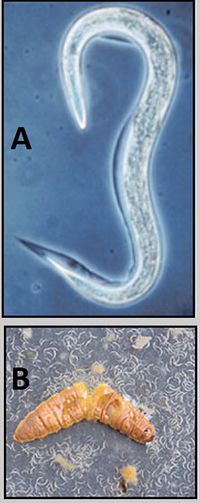
What specific kinds of microbes are typically involved in this interaction? Or associated with important processes? Describe key groups (genera, species) of microbes that we find in this environment, and any special adaptations they may have evolved to survive in this environment. List examples of specific microbes that represent key groups or are associated with important processes found in this environment. Add sections/subsections as needed. Look at other microbe listings in MicrobeWiki. Are some of the groups of microbes from your environment already described? Create links to other MicrobeWiki pages where possible.
Phases of X. nematophilus 1
X. nematophilus is believed to have phase I and phase II forms. Xn phase one motile. peritrichous flagella. Xn phase two non motile. but variable depending on environmental conditions.
Phase I 1a
Phase II 1b
The outer membrane OM of gram-negative bacteria performs many functions. They may be specialized to increase survival in adverse conditions. The OM has a mechanism for protein secretion, that in the case of bacterial pathogens will "inactivate" the host. establish. virulence factors are contained in OMV and excreted. Pseudomonas aeruginosa, Proteus mirabilis, and Serratia marcescens ---package enzymes (proelastases, hemolysins, protease. BACT. Bacteroides gingivalis has similar visicle properties. (toxicity, release toxins from OMV)
Other Microbes
photorhabdus (luminescens Xn related to photorhabdus (luminescens (homology of genese coding for the insecticidal proteins,associated with soil nematodes) Photorhabdus luminescens also has oral insecticidal activity
Nematode functions 2
Insect function 3
Species in the order Lepidoptera have numerous roles in an ecosystem. HOSTS: Lepidoptera. pest: Helicoverpa armigera. Pieris brassicae (cabbage white butterfly) larvae Manduca sexta. greater wax moth, Galleria mellonella.
Adult
Pollination, Dispersal Food source (bats, small mammals, carnivorious insects)
=Larval
Crop Damage, Agriculture (food source) Forest, tree damage (wood borers) - coleoptera though
other
potato/fungi Phtytophthora infestants
Microbial processes
What microbial processes are important for this microbial interaction? Does this microbial interaction have some ecosystem-level effects? Does this interaction affect the environment in any way? Describe critical microbial processes or activities that are important in this interaction, adding sections/subsections as needed. Look at other topics in MicrobeWiki. Are some of these processes already described? Create links where relevant.
Bacterium Functions 1
Toxic proteins
morgan et al. there is a DNA region of Xn found to encode for insecticidal protein, chitinase, and other pathogenic attributes. This suggests that the toxins of Xn are made up of multiple components. (multiple polypeptides)
TOXINS: the particulate fraction of the insecticidal proteins are more active than the soluable fraction (high-speed centrifugation). "Heating OMVs at 80 C for 15 min inactivated the insecticidal activity"When treated with a proteinase, up to 80% of the OMV proteins were degraded and inactive.
Outer Membrane Vesicles 1a
OMV emanate from the cell, encapsulate toxic proteins- protects proteins from degradation by the cells enzymes. vesicles are like a delivery system for the cell. separated from the cytosol and in most organisms is separated by at least one lipid bilayer. During growth, OMV are sloughed off from the surface of a cell. OMVs for Sn contain electron-dense compounds, proteins, porins, Abiosis excretions the bacteria produce antibiosis in vivo (in an organism) and in vitro (in artificial) Nutrient excretions
Nematode Functions 2
Nematodes are classified as part of the microfauna in a soil environment.Food web studies show that fauna contribute up to 30% of the total net nitrogen mineralization, mainly from microbial-feeding nematodes and protozoa. At this level of the soil ecosystem, nitrogen and other nutrients that would otherwise be locked up in organic form, are released through the decomposition activities of the microfauna. The possible Net Primary Productivity of the system is increased. Specific to the interaction between X. nematophilus and S. carpocapsae, the insect carcass is consumed by the nematode and the bacterium, efficiently using all available nutrients. The symbiotic relationship between the two organisms allows for maximum uptake or release of nutrients. Termite have microbial symbionts in the termite gut, and together the two organisms decompose ligno-cellulose.
Current Research
Biological Control
Agriculture. the most successful insecticide based on a microbe is that from the bacterium Bacillus thuringiensis. issue: resistence. so look for new genes. The genes must be orally active protein toxins. Such that the gene can be genetically engineered to be expressed in the plant tissue eaten by the pests. This is important because then only the insect species that are damaging the crops will be killed. This allows for safer pesticide use in cultivated fields, and it allows for greater biodiversity.
What factors allow for the bacteria to recognize environment?
The bacteria must have some mechanism by which to recognize whether it is in it’s host environment and regulate gene expression accordingly. ===What are the specific functions of the proteins in the outer membrane vesicles? unable to determine the toxicity individual proteins present in OMV mixtures for Xn. They confirmed that the OMV contain insecticidal protein toxins. Chitinase is likely to be an OM protein, but its effectiveness is not known. The presence of chitinase increases the pathogenic possibilities of the OMV compounds for Xn.
References
1. Floyd L. Inman and Leonard Holmes, 2012. Effect of Heat Sterilization on the Bioactivity of Antibacterial Metabolites Secreted byXenorhabdus nematophila. Pakistan Journal of Biological Sciences, 15: 997-1000.
1. H. Fossing, V.A. Gallardo, et. al. "Concentration and transport of nitrate by the mat-forming sulphur bacterium Thioploca". Nature. 1995. Volume 374. p.713-715. 2. Codispoti, L.A. et al. "High Nitrite Levels off Northern Peru: A Signal of Instability in the Marine Denitrification Rate". Science. 1986. Volume 233. p.1200-1202. 3. Schulz, H., Jorgensen, B., Fossing, H., and Ramsing, N. "Community Structure of Filamentous, Sheath-Building Sulfur Bacteria, Thioploca spp., off the Coast of Chile". Applied and Environmental Microbiology. 1996. Volume 62. p. 1855-1862. 4. Lauterborn, R. "Eine neue Gattung der Schwefelbakterien (Thioploca Schmidlei nov. gen. nov. spec.)". Ber. Dtsch. Bot. Ges. 1907. Volume 25. p.238–242. 5. Gallardo, V. A. "Large benthic microbial communities in sulphide biota under Peru-Chile Subsurface Countercurrent". Nature (London). 1997. Volume 268. p.331–332. 6. Strohm, T., Griffin, B., Zumft, W., and Schink, B. "Growth Yields in Bacterial Denitrification and Nitrate Ammonification". Applied and Environmental Microbiology. 2007. Volume 73(5). p. 1420-1424. 7. Mußmann, M., Hu, F., Richter, M., et al. "Insights into the Genome of Large Sulfur Bacteria Revealed by Analysis of Single Filaments". PLoS Biology. 2007. Volume 5(9). e230. 8. Macalady, J., et al. "Dominant Microbial Populations in Limestone-Corroding Stream Biofilms, Frasassi Cave System, Italy". Applied and Environmental Microbiology. 2006. Volume 72(8). p. 5596-5609. 9. Jørgensen, B. and Gallardo, V. "Thioploca spp. filamentous sulfur bacteria with nitrate vacuoles". FEMS Microbiology Ecology. 1999. Volume 28(4), p. 301-313. 10. Teske, A., Ramsing, N. B., Kuver J., and Fossing, H. "Phylogeny of Thioploca and related filamentous sulfide-oxidizing bacteria". Syst. Appl. Microbiology. 1996. Volume 18. p. 517-526. 11. Larking, J., and Strohl, W. "Beggiatoa, Thiothrix, and Thioploca". Ann. Rev. Microbiology. 1983. Volume 37. p. 361-362. 12. Schulz, Heide, and Schulz, Horst "Large Sulfur Bacteria and the Formation of Phosphorite". Science. 2005. Volume 307. p. 416-418.
Edited by <Chloe M. Mattia>, a student of Angela Kent at the University of Illinois at Urbana-Champaign.