Shower Curtain
Description of Niche

Location of Niche
Biofilms thrive in moist environments, including in our own households. One household alone is home to billions of microbes. A shower curtain houses many microbes, fungi, and potentially pathogenic organisms as well. Both the water-facing side of the shower curtain and the back side of it contains many microbes, which may circulate throughout the entire bathroom. Although it seems that the shower curtain doesn't provide microbes with a nutrient rich environment, microbes have certain adaptations in their lifestyle and metabolism that enable them to live in such an environment. In addition, the biofilm communities found on shower curtains are also found in environments with similar conditions, such as recirculating water systems, water pipelines, drinking water distribution systems, catheters, toilet bowls, pools, and hot tubs (even water filters).
Physical Conditions
A shower curtain will have a wide range of physical conditions depending on its location and usage. Typically, the temperature and pH will be highly variable- changing with every use of the shower as well as its location globally- while pressure remains relatively constant at near atmospheric pressure and moisture/humidity, although not constant, are relatively high. There is a wide range of organic and non-organic material that organisms must make use of or protect themselves from such as dead skin cells, soil, other organisms introduced, detergents, cleaning solutions, and possibly blood, urine, and feces. Thus, we see that the organisms that live in this niche have a unique metabolism that allow them to break down many materials and have several defense mechanisms to shield themselves from products that threaten them.
Influence of Surface Material, Sub-Niches and Adjacent Communities
Shower Curtain Surface Material
There are many factors which can either help microbes thrive on shower curtains, or make it harder for them to colonize. The material of the shower curtain, for example is one factor. Through observations, vinyl shower curtains have been known to cause biofilms the fastest. It is not known why, but it is probably due to the easy-attachable surface, and the number of folds that it causes in comparison to nylon shower curtains. The folds of a shower curtain make it a better habitat for many bacteria, such as Sphingomonas and Methylobacterium, to name a couple, which thrive in moist environments. The folds in these shower curtains are home to microbes with optimal growth at constant moist environments. If shower curtains are necessary, it is advised to use a nylon curtain, or even install a glass shower door, since the glass material accumulates the biofilms slower than by using a shower curtain.


Sub-niches
The specific location on a shower curtain greatly influences the type of microbe that will form.
As conducted in a documented 2004 Experiment by Scott T. Kelley and his colleagues, different areas of a vinyl shower curtain were sampled and analyzed. From their samples, they observed that different organisms were growing in different locations on the curtain. For example, from dry shower curtain samples, they noted the samples had white flakes, whitish pink flakes or pink flakes- increasing in the pink color as the bottom of the shower curtain was reached, or was most recently wet. These flakes turned out to be dried biofilm. While observing the folds of the shower curtain that were continuously wet, pink or pinkish orange films were seen. Although not directly stated in the experiment where each type of bacterium was located specifically, with the observations of the color of the bacterium, we could make predictions of which genus may be the most prevalent in these sub-regions of the shower curtain. For example Methylobacterium can be pink, yellow, or orange in color, while Sphingomonas can also appear as a white color (see chart below)- two bacteria that were found to have the highest concentrations on the shower curtain. These biofilms can thrive on the shower curtain because it feeds off of Carbon sources such as: dead human skin cells and soap, two things easily accessible for these microbes on the shower curtain.
Adjacent Communities

Microbes found on shower curtains also have other ways of being transported. In fact, toilets are also a source of pathogenic microbes that may appear on your shower curtain. When you flush your toilet, the bacteria may travel up to twenty feet away from your toilet. This can allow you to inhale these harmful pathogens while showering, and allow them to attach to either side of your shower door or shower curtain.
A major factor in the presence of certain pathogens in your toilet is the water system. The journal article, Microbiological Investigations of Rainwater and Graywater Collected for Toilet Flushing describes an experiment performed to see whether Rainwater, Graywater, or Waterworks Systems play a role in the amount of bacteria found in the toilet. Results show that in Rainwater Systems, there is a larger amount of microbes found within the toilet such as Aeromonas sp., Pseudomonas aeruginosa, Legionella non-pneumophila, Campylobacter jejuni, Mycobacterium avium, and Cryptosporidium sp. Waterworks systems did not show these pathogens in the toilet water. Graywater Systems showed a larger amount of Escherichia coli and Enterococcus than evident in Waterworks systems. (Albrechtsen 2002). Therefore, depending on the type of water system in a given household, this can potentially increase or decrease the number of pathogens found in your toilet. The lesser amount of pathogens housed in your toilet, the less possibility it is for the bacteria to latch onto the shower curtain, when you flush the toilet.
Conditions Under Which the Environment Changes
Shampoo: Shampoo is a hygienic product that is use on ones hair to remove dirt, oil, human particle and environmental debris. Shampoo producer enhance the products with vitamins and amino acids. As these products are used, it leaves trace amounts in the shower and on the shower curtain. These trace amounts of nutrients can be utilized by microbes.
Hair conditioner commonly includes ingredients such as long chain fatty alcohols (oleyl alcohol and stearyl alcohol), humectants (moisturizers, like panthenol), and silicone oils such as dimenthicone and cyclomethicone. While some components also include chlorides and other generally antibacterial chemicals, none of these are in high enough concentrations to damage bacteria through the protective matrix of the biofilm. Instead, these conditioning products may build up residue on shower curtains and provide a varied source of nutrients for the microbial community there.
Urine: As it turns out, if you can't hold the tinkle, and you urinate in the shower, you may be contributing to the populations of microbes that live on the shower curtain. Although urine typically has a lower pH than water- 5.0-8.0 versus 7.0 respectively (Bales 1984), urine contains many nutrients organisms can make use of. Human urine contains 6.2 mg of protein per every 100mL (Savory 1968) as well as small soluble DNA (Su 2004), giving microbes the substrates necessary for cell growth.
Microbes of the Shower Curtain Community
Many of the microbes present in shower curtains are opportunistic pathogens, and are therefore likely only to cause problems in immune-suppressed individuals. The most common and well-understood of these include methylobacterium, sphingomonas, mycobacterium, and serratia marcescens, though the first two are the most abundant (Kelley et al. 2004).

| Species | Description |
Methylobacterium
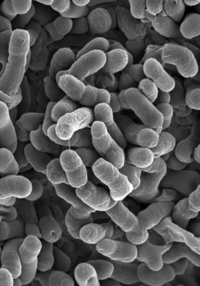 [1] Methylobacterium oryzae. |
Methylobacteria are non-motile, gram- negative, slow growing organisms belonging to the α-group of proteobacteria. They can be found in fresh water supplies, soil, dirt, various plant surfaces, and even as part of the human foot microflora. The members of methylobacteria, which includes many strains, are generally low virulence opportunistic pathogens, a threat only to immunocompromised individuals. The strains most commonly isolated from clinical samples are Methylobacterium mesophilicum and Methylobacterium zatmanii (Rice 2000). Another study shows that methylobacteria are opportunistic pathogens of low virulence than can cause mild clinical symptoms, such as fever, which can be treated by antibiotics (Hornei 1999). Sometimes known as pink-pigmented facultative methylotrophs ('PPFM's), their pigmentation can also be yellow or orange and is thought to offer UV protection. Methylobacteria are aerobes (they require oxygen to grow), and are unusual in that they are also facultative methylotrophs, or methane-oxidizing bacteria. Methylotrophs are able to grow by reducing carbon compounds that have one or more carbon atoms but no carbon-carbon bonds. Methylobacteria can metabolize methylamine, methanol, C2, C3, and C4 compounds, and even formaldehyde to use the resulting carbon as energy; this unique ability gives them an advantage in dense communities such as biofilm, where they are able to utilize nutrients that other bacteria are not. They are commonly found on the roots and leaves of plants, where they can metabolize the methanol emitted by the stomata of plants and are thought to produce a range of phytohormones, stimulating seed germination and plant development. As methylotrophs they are also found in habitats such as wetland rice fields, where methane is the end product of anaerobic degradation of organic matter (Eller and Frenzel 2001). There is no conclusive evidence, however, on whether these particular traits effect their participation in the biofilm community. It is also suggested that methylobacteria can nourish themselves by using products of other bacteria growing on humans, particularly on human feet. Certain bacteria, such as Brevibacterium linens, live on human feet and produce a variety of methylated sulfur compounds from the breakdown of amino acids, such as methanethiol from methionine, and other compounds common in dead skin cells. Methanethiol and dimethylsulphide are important intermediates in the biogeochemical sulphur cycle, and these products might also be used as substrates for the growth of methylotrophic bacteria like Methylobacterium podarium (Anesti 2004). Methylobacteria are often found in water purification and distribution systems, including water from dental units and blood bank purification units (Rice 2000). They can also be found automobile air-conditioning systems, printing paper machines, and other damp environments, and are common in tap water, as some strains exhibit resistance to chlorine. On shower curtains, they may contribute to the pink color of biofilm (Kelley 2004). |
Sphingomonas
 [2] Methylobacterium oryzae. |
One genus of bacteria that is found in the shower curtain niche is the Sphingomonas. These kinds of gram- negative bacteria are able to metabolize and live off of a wide variety of compounds, as well as contaminants, that are naturally occurring in the shower/ shower curtain area. Sphingomonas are able to use a broad range of carbon compuds including dibenzofuran and hexachlorohexane. Sphingomonas are also capable of degrading aromatic compounds. They are the most abundant of the biofilm organism detected in this niche. In addition to the shower curtain area, Sphingomonas have been frequently isolated from the soil, industrial water, as well as in sediments. Some of the deep surface Sphingomonas also isolate and metabolize aromatic compounds such as toluene and naphthalene. However laboratory strains have not shown these properties. Sphingomonas paucimobilis is an example of a specific bacterium of the genus. This was also found in the shower curtain area. It has a history of infecting immune compromised individuals. In addition it also affects persons with predisposing conditions. (Adams, W. E.,). Infections caused by this potential pathogen include urinary tract infections, pneumonia, cutaneous infections, and visceral abscesses. In hospitals, the infections of this bacterium have been traced to fluid humidifiers as well as tap water. (Scott T. Kelley). |
Mycobacterium
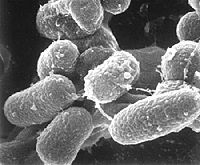 [3] Mycobacterium avium. |
Mycobacteria are commonly found in the bathroom environment as mycobacterium avium, subspecies hominis (MAH): part of a mycobacterium avium complex (MAC) that includes subspecies avium (MAA) and paratuberculosis (MAP). They can cause respiratory infections and also non-infectious respiratory problems when inhaled, but those found on shower curtains are mostly harmless to healthy individuals and are, instead, opportunistic pathogens that affect the immunosuppressed. Mycobacterium avium can also be found naturally in soils, plants, fish, drinking water and natural water, and as intracellular pathogens are also able to survive and grow in animal macrophages and phagocytic protozoa (Falkinhamm III 2004). Mycobacterium are uniquely and extraordinarily resitant. They can tolerate the extreme temperature of ice machines and water heaters, and are more than a hundred times more resistant to chlorine than is e. coli. Additionally, their ability to survive phagocytosis and grow within phagocytes and amoebas protects them from conventional water purification regimens (Cirilli et al. 1997) The mycobacteria's lipid-rich cell wall confers significant hydrophobicity, and in wet environments this encourages their attachment to surfaces. In terms of a biofilm, mycobacterium are thought to be among the first to colonize because of this trait (Steed and Falkinham III 2006). |
Serratia marcescens
 [4] Serratia marcescens, showing characteristic pinkish hue. |
Serratia marcescens is a motile, airborne, gram negative bacterium found naturally in soil, water, the subgingival biofilm of teeth, and sometimes in the intestines. In humans, it is a pathogen associated with a range of problems, including urinary tract infections, wound infections, conjunctivitis, keratitis, and meningitis. In bathrooms, it is commonly responsible for the red or pink slimy substance found on surfaces. S. marcescens prefers damp environments, and can grow in temperatures ranging from 5 to 40°C and and pHs from 5 to 9; additionally, it will grow anywhere phosphorous-containing materials or fatty substances accumulate, i.e., soap residues in shower areas, feces in toilets, food residues in pet dishes (Hejazi and Falkiner 1997). S. marcescens is notable for being able to perform casein-hydrolysis, which produces metalloproteases believed to function in extracellular matrix formation. Another distinction in its metabolism is the ability to break down tryptophan and citrate, and to convert the latter to a source of carbon. It is a facultative anaerobe and can produce lactic acid by oxidative or fermentative metabolism (Hejazi and Falkiner 1997). Only in recent history has S. marcescens been recognized as a human pathogen, and several strains are antibiotic-resistant. While chlorine is known to help control S. marcescens populations, the most effective disinfectant is bleach. |
Legionella
 [5] Legionella pneumophila. |
Legionella is a motile, gram negative aquatic bacterium found in creeks, streams, air conditioning systems, and other human water supply and distribution systems. In nature is flourishes as a parasite to eukaryotic cells and amoebae, and in humans it acts as an opportunistic pathogen, infecting immunocomprised individuals via inhaled aerosols. Legionella pneumophila is responsible for Legionnaires' disease and Pontiac fever. Legionella can survive in temperatures below 20°C and up to 55°C, and can be eradicated by persistent treatment with chlorine or exposure to high temperatures. (Swanson, 2000) |
Additional Bacteria
A large number of other microbes have been found to live on shower curtains, including but not limited to: Afilpia felis, Moraxella osloensis, Actinomycetales, Nocardia, Gordonia (Kelley 2004), Methicillin Resistant Staphylococcus aureus (MRSA), and Escherichia coli (Barrett 2003).
Shower Curtain Fungi)
Numbered ticks are 11 µM apart.
Photograph by Bob Blaylock.
| Species | Description |
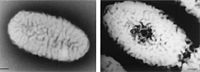
Aspergillus niger
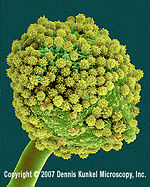 Aspergillus niger from Dennis Kunkel Microscopy, Inc. |
‘‘Aspergillus niger’’, also known as “black mould”, is a Eukaryotic fungi in the Ascomycota family (NCBI). Its optimal temperature range is between 28-34 ºC (Szewczyk 1994) although it can tolerate greater temperatures in both directions. It has been found to live in a variety of wet places including the shower curtain (Barret 2003) and rotting plants (Curtis 1974). |
| Phoma violacea | Phoma violacea is a Eukarotic fungi also in the Ascomycota family (NCBI). Its optimal temperature range is 22.5-25ºC, but it is able to tolerate 15-28 ºC (Eveleigh 1961). It is found in wet areas like the shower curtain (Green 1972), but has also been found to deteriorate paint on a variety of surfaces as well as dead animals. Its color varies from red to violet depending on the pH of the environment which it can tolerate and even change from the pH range of a little above 2 to above 8 (Eveleigh 1961). |
Microbial Interactions with Their Environment and Community
Microbes on shower curtains generally form biofilms, which allow participating bacteria to interact and cooperate in ways they normally would not. Bacteria in biofilm communities exhibit significant behavioral differences from their independent counterparts, and their cooperation results in greater resistance to environmental stressors. One prime example is the production of EPS (extracellular polymeric substance or exopolysaccharide) by cells in a biofilm; this sticky polysaccharide matrix holds the cells together, attaches them to surfaces, and facilitates biochemical communication between cells. Most importantly, the matrix offers the bacterial community within a great deal of protection against detergents and antibiotics, making biofilms very difficult to destroy completely (Kelley et al. 2004, Costerton et al. 1999, Davies et al. 1998).

Some strains of methylobacterium have been shown to stimulate seed germination and plant development by production of chemical signals such as cytokinin zeatin, indole acetic acids, and auxins- all of which act as phytohormones, or plant growth hormones (Ivanova et al. 2001, Lidstrom and Chitoserdova 2002). In their natural environment, this gives rise to a mutually beneficial relationship between host and bacteria, but in shower curtains the effect is as yet unknown.
Methylobacteria are also known to fix nitrogen, and certain end products of their unique metabolism are thought to serve as regulators or signals in bacterial communities. For example methylobacterium extorquens AM1 produces several acyl-homoserine lactones, important regulatory molecules for many gram-negative bacteria (Penalver et al. 2006). Additionally, methylobacterium are known slime-producers, and contribute in large part to the production of EPS in biofilms; this is one reason for their abundance in the shower curtain biofilm community, and why they are considered among biofilm settlement "pioneers". Methylobacterium capable of utilizing toxic halogenated methane derivatives as sources of carbon can also serve to protect their biosphere from those toxic pollutants.(Trotsensko and Doronina, 2003)
Another genus of bacterium also common to the shower curtain niche is the Sphingomonas. There is still research being done as to how these two genuses interact with each other. No current evidence of symbiotic or negative competition is available. However, due to the sacristy of the nutrients available and also the type of nutrients present in the niche, it can be hypothesized that some sort of negative competition might exist (microbewiki). The Sphingomonas found in the shower curtain niche do not produce and antifugals or antibiotics (Scott T. Kelley).
Mycobacteria are also considered biofilm pioneers, as their highly hydrophobic membranes and resistance to chlorine allow them to be among the first to colonize, attaching to surfaces and providing the groundwork for future biofilm growth (Steed and Falkinham III, 2006).
The fungus known to grow in the shower curtain has several methods to compete with other organisms. For example, Aspergillus niger is able to produce the antibiotics malformin and tensyuic acids (Curtis 1974 and Yoko 2007), as well and antifungal peptides (Gun 1999). Phoma violacea, in contrast, can compete by dramatically decreasing the pH of the environment (Eveleigh 1961) making it difficult for other organisms to thrive.
Microbial Metabolism
Methylobacterium is a facultative methylotroph, which means it has the ability to grow by reducing carbon compounds that have one or more carbon atoms but no carbon-carbon bonds. It can grow on methylamine, methanol, C2, C3, and C4 compounds, including the methanol emitted by the stomata of plants, and even by metabolizing formaldehyde(Eller and Frenzel 2001).
The fungal species Phoma violacea is able to digest a variety of carbohydrates such as: glucose, mannose, galactose, sucrose, lactose, raffinose, xylose, rhamnose, mannitol, dextrin, starch; as well as a wide variety of fatty acids including: laurate, myristate, palmitate, stearate, linoleate, ricinoleate, "alkali-refined linseed oil"; and is vitamin-autotrophic (Eveleigh 1961). Thus, they are well adapted to changing conditions of the shower because they are able to metabolize a wide range of materials and create molecules that are essential to them.
Sphingomonas are aerobic diethyl phthalate (DEP) degrading bacterium. They are able to degrade monoethyl phthalate (MEP), dimethyl phthalate (DMP), and dibutyl phthalate (DBP), and diethylhexyl phthalate (DEHP). These phthalates are used as additives in plastics, celluloses, and polyvinyl acetates. They are present in the material of shower curtains. Studies have shown that the bacterium grow well at pH 7 and at temperatures that range from 25 – 35 degrees Celcius. There is evidence showing that the degradation of DEP and the other phthalates needed a lag time of at least 30 to 50 hours at these optimal conditions. The degradation rate also appeared to be concentration dependent that caped at 14 milligrams. (Fang, H. H).
Preventing and Fighting Shower Curtain Bacteria
Antimicrobial Shower Curtain
Aegis™ Environments have developed antimicrobial materials that include shower curtains.(Aegis Environments, 2008) This “Microbe Shield Technology” kills microbes without the use of harmful chemicals that are conventionally used. The compound 3-Trimethoxy silyl propyl dimethyl octadecyl ammonium chloride is responsible for its antimicrobial properties. There are three parts to this compound. First, the silane base is the anchor for this antimicrobial compound. It is covalently bound to the surface by hydrolysis reactions which allow crosslinking and polymerization to other molecules. Second, the centrally positively charged nitrogen draws the microbes (cell membrane is negatively charged.) Third, the long molecular chain (18) is responsible for piercing the microbe. As the microbes are drawn to the positively charged nitrogen, they are pierced. Electrocution also occurs due to the interaction of the positive nitrogen and the negative cell wall.
Shower Cleaners

Shower cleaners are often used to clean shower curtains. Shower cleaner usually contains "a nonionic surfactant, a chelating agent, and an alcohol."(Rouhi, 2001) Most showers cleaners’ active ingredients are: isopropyl alcohol; Antarox BL-225 (nonionic surfactant); and Hamp-ene diammonium EDTA (chelating agent.) The alcohol component assists in dissolving the materials and fatty substances (soap and oily substances) in the water. The chelating agent segregates ions and pulls them into solution. Finally, the nonionic surfactant breaks the surface tension of the water. This allows the water to glide down the curtain effortlessly. Thus removing the majority of possible nutrients the microbes could use. (Rouhi, 2001)
Bleach

Bleach is a sodium hypochlorite solution that is extremely efficient in eradicating microbes. It is a cheap and efficient disinfectant. Sodium hypochlorite enters the cell, interacts with the microbes components and compromises the cell. It exchanges atoms with other compounds, such as enzymes in bacteria and other cells. This results in cell death. For this reason, it’s a good sanitizer. Lenntech, 2008
Chlorinated Water
In the US, water is often treated with chlorine in order to disinfect and purify. Chlorine is a strong oxidant that will oxide the DNA of all living matter. This bleaching effect causes major protein damage resulting in the lost of function. Because of its toxic effect on harmful microbes, chlorine is used as a universal treatment for water sources. This means chlorinated water is used in showers, thus providing some disinfecting effect to the shower curtain. However its viability is about thirty minutes. Lenntech, 2008
Current Research
Molecular Analysis of Shower Curtain Biofilm Microbes
Scientists Scott T. Kelley, Ulrike Theisen, Largus T. Angenent, Allison St. Amand, and Norman R. Pace conducted experiments with regards to the type of microbe present on typical household shower curtains. Vinyl shower curtains are excellent homes to these microbes due to their varying areas on the shower curtain that the organisms have adapted to. Some areas are constantly moist, and some areas form “soap scum”, leaving a whitish, dry and flaky residue on a vinyl shower curtain. These scientists wanted to identify and analyze the presence of microbes and their toxicity, if any, on a household shower curtain.
Four different shower curtains were used to take samples from varying environments, that were at least six-months old, and frequently used. The first curtain contained samples from the bottom of the curtain (described as white-pink flakes), which was dry and also a sample from the “fold” of the shower curtain, which was moist and pink in color. The second curtain sample was from a dry shower curtain that had white flakes. The third curtain had a sample of pink flakes from the bottom area, and the fourth curtain had a sample from a constantly moist curtain which had pink-orange colored biofilm.
After taking these samples, the scientists held their environmental conditions under careful watch, and began conducting a series of experiments such as: Epifluorescence microscopy, DNA extraction, Polymerase Chain Reaction (PCR), Cloning and Restriction Fragment Length Polymorphism (RFLP) Analysis, and finally sequencing. What they found was that there two main families of bacteria— Methylobacterium and Sphingomonas with many different species identified and others that were predicted to be evident as well. Most of these bacteria are not pathogenic, but some do have a history of infecting immune-suppressed individuals. In addition, they can feed off of carbon sources in the shower such as dead skin cells, soap, and other “bath area volatiles”. (Kelley 2004)
Microbiological Investigations of Rainwater and Graywater Collected for Toilet Flushing
The use of certain water systems tends to influence the presence of microbes and pathogens in household water usage. Scientists sought out three types of water systems: Rainwater System, Waterworks System, and Graywater System, and determined how these types of water systems impacted the number of microbial communities evident in toilets.
Scientists performed a series of experiments by measuring the “microbial quality” of the water—which essentially measures the number of bacteria present in the sample. For the Rainwater System samples, scientists took seven samples from rainwater storage tanks and from the toilet bowl. The Graywater System samples were taken from four plants that UV-radiated the water for toilet flushing. The Waterworks samples were used as reference samples to compare the microbial differences between these other types of water systems.
After the experiments were performed, the results showed that Rainwater Systems helped increase the amount of microbial activity in toilet water. Collectively in the Rainwater system samples, the following microbes were found: Aeromonas sp., Pseudomonas aeruginosa, Legionella non-pneumophila, Campylobacter jejuni, Mycobacterium avium, and Cryptosporidium sp. In comparison to the reference samples from the waterworks toilets, it showed that “the use of rainwater introduced new, potentially pathogenic microorganisms into the households” that were not present in the waterworks toilet water samples. The Graywater Systems showed only a larger number of Escherichia coli and Enterococcus (Albrechtsen 2002).
Cultivation-Independent Characterization of Methylobacterium Populations in the Plant Phyllosphere by Automated Ribosomal Intergenic Spacer Analysis
Scientists Claudia Knief, Lisa Frances, Frank Cantet, and Julia A. Vorholt conducted experiments to gain better insight into the distribution of different Methylobacterium species in diverse ecosystems. The reason behind their experiment was the fact that this genus of bacteria were widespread in the environment, however, the role of them in their ecology such as the plant phyllosphere were not very well known.
They took several samples from different plant species and studied them using a rapid and specific cultivation-independent method using 16S rRNA gene-targeted primers specific for this genus. These primers were combined with a reverse primer that binds to the tRNA gene located upstream of the 23S rRNA gene in the 16S-23S intergenic spacer (IGS)in PCR. They used both primers to amplify DNA from Phyllosphere plant in order to evaluate primer specificity. They developed a method called ARISA (a fast and high-resolution fingerprinting method) in order to differentiate Methylobacterium strains.
Generation of fingerprints of Methylobacterium communities by ARISA collected from phyllosphere samples, enabled them to compare these communities of Phyllosphere on leaves of different plant species. They found out that the 16S-23S rRNA IGS sequence was more useful to differentiate similar strains than the 16S rRNA. What they observed from this experiment was that some of the plant species had very similar Methylobacterium communities. This differed from those of other plant species grown at the same sampling site, and yet, leaves of some other plant species harbored different communities. The results of this study indicate that ARISA is a well-suited tool for characterization of Methylobacterium communities in environmental samples. (Knief 2008).
Chlorination of model drinking water biofilm: implications for growth and organic carbon removal
Phillip Butterfield, Anne Camper, Brian Ellis and Warren Jones conducted experiments to see the effect of chlorine on biofilm that are typical in drinking or industrial water. With complex water treatment, the ability of biolfilm formation in water pipelines with the presence of disinfectants such as chlorine still occurs. In their experiment, they aspire to obtain a more basic understanding of the effect of chlorine on the biofilm. They will accomplish this by compared biomass and kinetic parameters in the chlorinated reactors to those of non-chlorinated reactors. These reactors were operated using three substrates: amino acids; carbohydrates; and humic substrates. The chlorine reactor maintain a free chlorine residual of 0.09-0.15 mg/L. The observed yield values were less in the chlorinated reactor when compared to the control. However specific growth and the rate at which carbon is removed were higher. The impacts of the different substrates also affect the growth. The carbohydrate substrate had the greatest difference, while the amino acid was almost the same. These findings can now be applied to disinfectant the water treatment systems more efficiency. (Butterfield, 2002)
Cadmium biosorption by Sphingomonas paucimobilis biomass
Sphingomonas paucimobilis is a mocrioorganism that has a good tolerance to cadmium. Cadmium is a metal that is used in the industrial plants all over the world. Its toxicity has made it one of the leading sources of contaminants in the environment. The biosorption and tolerance properties of S. paucimobilis to this metal was studied in the laboratory.
Three microorganisms were taken from samples of wastewater from a municipal treatment plant. One of them was S. paucimobilis. They were brought to a laboratory and cultured in different nutrient media that contained different cadmium concentrations. These different samples were collected and filtered throught membrane filters. The filtered cadmium samples that were filtered were analyzed.
Results of the study clearly showed that S. paucimobilis was the most tolerant to the cadmium metal. However, too much cadium prevented its growth (about 25-200 mg/l). At a pH level of 6, this species can effectively remove cadmium. This study shows that using bacteria in the future can be an effective and ecofriendly to celan up toxins.(Tangaromsuk, J)
Summary
Bacterial communities found on shower curtains bear many resemblances to the communities found on indwelling medical devices, water processing and distribution systems, and in other damp corners around the home. An important attribute these communities have in common is their ability to support biofilms, diverse communities of microbes living in fortresses of polymeric matrix and attached to surfaces. The biofilm of the shower curtain is home to such microbes as Sphingomonas, Methylobacteria, Serratia, Legionella, Mycobacteria, Apergillus niger, and Phoma violacea, among others. Many bacteria on the shower curtain are pathogenic but of low virulence, and only a threat to individuals with compromised immune systems. They are most commonly inhaled as airborne spores and pose the greatest threat to people in hospitals.
Their environment provides nourishment in the form of dirt, grime, and cells washed from the human body, and even from shower product leftovers, any of which may become trapped in the folds of the shower curtain or upon the biofilm matrix. The microbes on shower curtains are well-adapted to their environment because they have an efficient metabolism of many substrates, can form spores when nutrients are unavailable, and may exhibit some degree of resistance to the chlorine and hard metals found in tap water. Many are also tolerant of high temperatures as well as the mild cleansers in shower products such as shampoo and conditioner, and even organisms who have no mechanism to tolerate cleaners are given protection from the biofilm matrix.
Getting rid of shower curtain bacteria requires persistence and regularity. Shower cleaners fight biofilms by washing away their nutrients more efficiently, while bleach and chlorine are commonly used disinfectants that target the bacteria directly. Recently, there have also come to light options such as anti-microbial shower curtains. Much of the current research, additionally, is directed towards the removal and eradication of shower curtain bacteria.
References
Adams, W. E., M. Habib, A. Berrington, R. Koerner, and D. H. Steel. "Postoperative Endophthalmitis Caused by Sphingomonas paucimobilis." JOURNAL OF CATARACT AND REFRACTIVE SURGERY. 32. 7 (2006): 1238-1240.
Anesti, Vasiliki, Jyotsna Vohra, Shalini Goonetilleka, Ian R. McDonald, Bettina Straubler, Erko Stackebrandt, Donovan P. Kelly, and Ann P. Wood. 2004. "Molecular detection and isolation of facultatively methylotrophic bacteria, including Methylobacterium podarium sp. nov., from the human foot microflora." Environmental Microbiology. Blackwell Publishing Ltd.
Azeredo, J., and R. Oiiveira. "The Role of Exopolymers Produced by Sphingomonas paucimobilis in Biofilm Formation and Composition." BIOFOULING -CHUR-. 16 (2000): 17-28.
Azeredo, J., and R. Oliveira. "The Role of Exopolymers in the Attachment of Sphingomonas paucimobilis." BIOFOULING -CHUR-. 16 (2000): 59-68.
Bales JR, Higham DP, Howe I, Nicholson JK, Sadler PJ. "Use of high-resolution proton nuclear magnetic resonance spectroscopy for rapid multi-component analysis of urine." Clin Chem. 1984 Mar;30(3):426-32.
Barrett Tony D. International Journal of Therapy and Rehabilitation, Vol. 10, Iss. 6, 01 Jun 2003, pp 281.
"Chlorine as Disinfectant for Water." Lenntech Disinfectants. 2008. Lenntech. 28 Aug. 2008.
Cirillo, JD, S Falkow, LS Tompkins and LE Bermudez , "Interaction of Mycobacterium avium with environmental amoebae enhances virulence." Infect. Immun., Sep 1997, 3759-3767, Vol 65.
Curtis, Roy W., Walter R. Stevenson, and John Tuite. "Malformin in Aspergillus niger-Infected Onion Bulbs (Allium cepa)." Appl Microbiol. 1974 September; 28(3): 362–365.
Eller, G. and P. Frenzel. 2001. “Changes in activity and community structure of methane oxidizing bacteria over the growth period of rice.” App. Environ. Microbiol. 67, 2395-2403.
Eveleigh, D. E. "The growth requirements of Phoma violacea, with reference to its disfiguration of painted surfaces." Ann. appl. Biol. (1961), 49, 412-423.
Falkinham JO 3rd, Iseman MD, de Haas P, van Soolingen D., "Mycobacterium avium in a shower linked to pulmonary disease.", J Water Health. 2008 Jun;6(2):209-13.
Fang, H. H., D. Liang, and T. Zhang. "Aerobic Degradation of Diethyl Phthalate by Sphingomonas Sp." Bioresource Technology : Biomass, Bioenergy, Biowastes, Conversion Technologies, Biotransformations, Production Technologies. 98. 3 (2007): 717-720.
Fordham von Reyn, C., Maslow, J.N., Barber, T.W., Falkinham, J.O., "Persistant colonisation of potable water as a source of Mycobacterium avium infection in AIDS", Lancet, Vol. 343, 1137-1141 (1994).
Green W. F. "Precipitins against a fungus, Phoma violacea, isolated from a mouldy shower curtain in sera from patients with suspected allergic interstitial pneumonitis." Med J Aust. 1972 Apr 1;1(14):696-8.
Gun Lee D, Shin SY, Maeng CY, Jin ZZ, Kim KL, Hahm KS. "Isolation and characterization of a novel antifungal peptide from Aspergillus niger." Biochem Biophys Res Commun. 1999 Oct 5;263(3):646-51.
Hornei, B., E. Luneberg, H. Schmidt-Rotte, M. Maass, K. Weber, F. Heits, M. Frosch, and W. Solbach. 1999. "Systemic infection pf and immunocompromised patient with Methylobacterium zatmanii." J. Clin. Microbiol. 37:249.
Maliti, Charles M., Dominick V. Basile, and William A. Corpe. "Effects of Methylobacterium spp. strains on rice Oryza sativa L. callus induction, plantlet regeneration, and seedlings growth in vitro." The Journal of the Torrey Botanical Society, Volume 132, Issue 2 (April 2005), pp. 355–367.
Nieto Penalver ,C ., D . Morin , F . Cantet , O . Saurel , A . Milon , J . Vorholt. "Methylobacterium extorquens AM1 produces a novel type of acyl-homoserine lactone with a double unsaturated side chain under methylotrophic growth conditions." FEBS Letters , Volume 580 , Issue 2 , Pages 561 - 567.
"Research on microbial biofilms (PA-03-047)". NIH, National Heart, Lung, and Blood Institute (2002-12-20).
Rice, E. W., D. J. Reasoner, C. H. Johnson, and L. A. DeMaria, "Monitoring for Methylobacteria in Water Systems." Journal of medical microbiology, Nov.2000, P.4296-4297.
Savory, John, Pin H. Pu 1, and F. William Sunderman Jr. 1 "A Biuret Method for Determination of Protein in Normal Urine." Clinical Chemistry, Vol 14, 1160-1171, 1968.
Serratia marcescens. http://www.serratia-marcescens.org/
Su, Y. H., M. Wang, D.E. Brenner,A. Ng , H. Melkonyan, S. Umansky, S. Syngal, T. M. Block. "Human urine contains small, 150 to 250 nucleotide-sized, soluble DNA derived from the circulation and may be useful in the detection of colorectal cancer." J Mol Diagn. 2004;6:101–7.
Szewczyk, K. W., L. Myszka. The effect of temperature on the growth of A. niger in solid state fermentation. Bioprocess Engineering lo (I994) 123-126.
Tangaromsuk, J., P. Pokethitiyook, M. Kruatrachue, and E. S. Upatham. "Cadmium Biosorption by Sphingomonas paucimobilis Biomass." Bioresource Technology : Biomass, Bioenergy, Biowastes, Conversion Technologies, Biotransformations, Production Technologies. 85. 1 (2002): 103-105.
Verhoefa, René, Pieter de Waardb, Henk A. Scholsa, Matti Siika-ahoc and Alphons G. J. Voragen. "Methylobacterium sp. isolated from a Finnish paper machine produces highly pyruvated galactan exopolysaccharide." Carbohydrate Research, Volume 338, Issue 18, 1 September 2003, Pages 1851-1859.
Yoko Hasegawa, Takashi Fukuda, Keiichi Hagimori, Hiroshi Tomoda and Satoshi Ōmura, “Tensyuic Acids, New Antibiotics Produced by Aspergillus niger FKI-2342.” Chem. Pharm. Bull., Vol. 55, 1338-1341 (2007).
edited by Mary De Unamuno, Christy Furukawa, Raymon Araniego, Harn Chiu, Coel Momita, and Delaram Rostami (students of Rachel Larsen)
