Carbon cycle
Introduction
Soil stores at least three times as much carbon (in soil organic matter or SOM) as is found in either the atmosphere or in living plants (Fischlin, A, et al, 2007). A thorough understanding of the soil carbon cycle is essential to understanding the global carbon cycle, and “to the stewardship of ecosystem services provided by soils, such as fertility, water quality, resistant to erosion and climate mitigation through reduced feedbacks to climate changes(Schmidt, et al, 2011).” Atmospheric carbon, usually CO2, is brought into the biosphere by plants, where the carbon is fixed into various organic compounds. Soil microbes use the various components in plant residue as a source of energy and carbon for biosynthesis. This process is referred as decomposition. The easily degradable fraction of plant residue is turned into CO2 and microbial biomass quickly. As time goes, the intermediate fraction is also turned into CO2 and microbial biomass. The decomposition of the resistant fraction can take years, decades, or even centuries. All carbon compounds are eventually turned back into CO2, and released into atmosphere through microbial respiration and other processes. While complex carbon compounds are broken down into their constituents, new SOM is forming in the soil. Recent research has confirmed the importance of microbes in the formation of SOM. Microbes are the eye of the needle---carbon has to be filtered through for SOM to be formed.
The Carbon Cycle
Carbon Fixation
An important step of the carbon cycle is the fixation of atmospheric CO2 and its subsequent assimilation into organic molecules. Organisms that can convert CO2 into organic molecules are called autotrophs, and include plants, algae, some bacteria, and some archaea. Some bacteria specifically involved in carbon fixation are Cyanobacteria (such as Chroococcus, Nostoc, and Spirulina) and some species of Aquifex,such as Aquifex aeolicus. Cyanobacteria in the soil are also important Nitrogen fixers in the Nitrogen cycle including GHG.
Decomposition of Organic Matter and the Microbes Involved
Decomposition of organic residue is one of the major functions of microorganisms in the soil. The soil microorganisms utilize the residue components for energy and carbon source to synthesize the new cells. The organic materials added to soil are primarily plant residues from either cultivated crops or native vegetation, which are the source of energy stored in the carbon compounds. The crop agricultural ecosystems primarily include the combination of leaf, stem, and root tissues remaining after harvest. On the other hand, the residues that undergo decomposition in the natural ecosystems are derived from native prairie and forest vegetation. There are two types of plant residue that assimilate into the soil. One is above ground residue, which is derived from falling debris and other plant that are above the soil surface. The second type is deposited from root systems that complete their life cycle throughout the course of a particular season. These residue with the root exudates supply the substrate carbon input into the soil. The decay process of above-ground residue begin and initiate due to different agricultural management systems and practices. A portion of these substrate goes into microbial mass as it is used by the soil microorganisms for reproduction and metabolic activity and a larger part of it is lost as carbon dioxide due to the energy yielding metabolic processes of the microbial community. In the current study, Francesca Cotrufo emphasized that not all the litter Carbon loss are mineralized to carbon dioxide, but a part of these Carbon loss is transferred to mineral soil, where it contributes to the formation of soil organic matter through the two main pathways, dissolved organic matter-microbial path and physical transfer path (hyperlink to SOM path-reference M. Francesca Cotrufo).
Chemical Compounds
The composition of the crop residues added and decaying to soil are varied. Plant residues are comprised of many complex polymers such as lignin and cellulose and contains water-soluble organic compounds such as proteins, carbohydrates, and organic acids for the vast majority of microorganism in soil.
Proteins
Proteins are one of the most important components of plant residue added to soil. Protein is a polymers of amino acids linked by peptide bonds. A variety of microorganisms in soil can produce proteolytic enzymes such as protease and peptidase, which can break the peptide bond of the protein and hydrolyze it into individual amino acids. These amino acid monomers can then be utilized by microbes for either catabolism or for the synthesis of new essential proteins.(textbook)
Carbohydrates
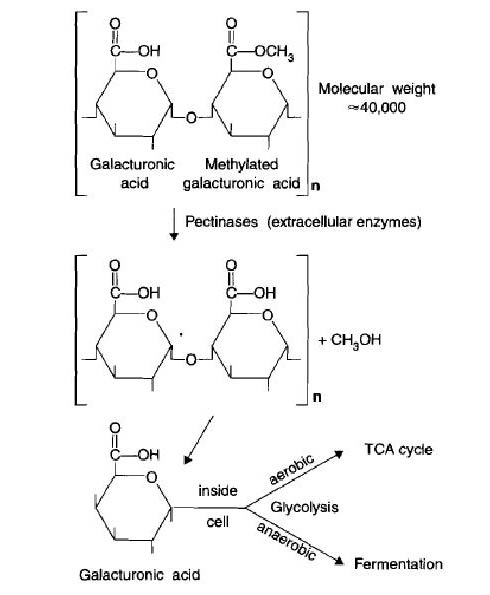
From 5-25% of soil organic matter is combined in the form of carbohydrates, which include simple sugars, cellulose, and hemicellulose. These carbohydrates are rapidly degraded by varied microorganisms in the soil including bacteria, archaea, actinomycetes, and fungi. During degradation soil microorganisms synthesize extracellular polysaccharides. These polysaccharides bind soil into water stable aggregates so that the aggregates are more permeable to water and air. Polysaccharides also affect the cation exchange capacity of soil and act as an energy source for other microorganisms. Heterotrophic microbes can easily metabolize simple sugars. Plants link glucose molecules (and other sugar monomers) into long chains to produce polymers such as cellulose, which requires more specialized organisms to degrade. For example: Starch is made of amylose (alpha 1,4 bonded glucose) and amylopectin (alpha 1,6 bonded glucose), and is relatively easy for most organisms to degrade. Cellulose, however, is a polysaccharide of glucose connected with beta 1,4 bonds, and is more difficult to degrade. No animal can degrade cellulose, so bacteria can frequently be found in mutualistic relationships with detritivores: the bacteria degrade the cellulose enough that the animal is able to digest it.
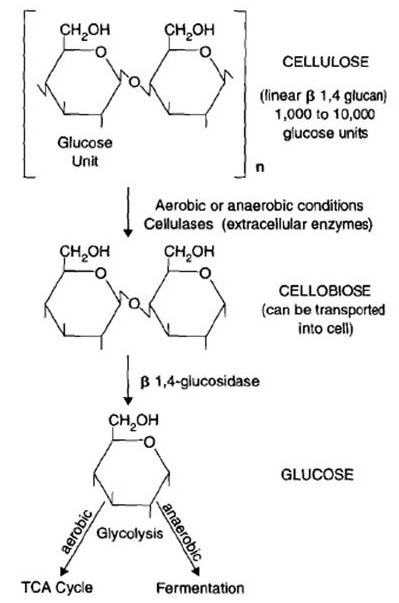
Cellulose
Cellulose is the most abundant carbon input in soil. It’s a structural polysaccharide that is made of 1400 to 10000 glucose units. In order to access the glucose monomer, the cellulose must be cleaved by extracellular enzymes. These pieces are then transported into the cell for energy generation (catabolism) or production of biomass (anabolism). Cellulose is used by a diverse group of soil organisms including fungi such as Penicillium and Aspergillus and bacteria such as Streptomyces and Pseudomonas. Fungi and bacteria are important participants in the extracellular cleavage of cellulose [2].
Hemicellulose
Hemicellulose is the next most common carbohydrate in plants. It is a branched polymer with varied sugar monomers (glucose, mannose, and galactose) and bonds. The decomposition of hemicellulose is similar to that of cellulose in that the initial depolymerization step takes place outside of the cell, and the sugars produced are then transported into the cell for catabolism or anabolism. Even though hemicellulose decomposition is much quicker than cellulose decomposition, cells will utilize simple sugars as substrates before hemicellulose [2].
Chitin
Chitin is a special compound which can be found in the integument of arthropods and the cell walls of fungi. Chitin is one of the cell-wall component of many common soil fungi (e.g., Aspergillus and Penicillium) as around 3% to 25% of these soil fungal biomass are chitin(textbook). Chitin is usually protected from degradation by the protein-chitin complex in its natural normal stage in soil. Due to this type of protection, chitin is an important component in soil organic matter formation. The polymer is not easily degraded and requires a variety of enzymes to do so. The dominant chitin degraders are the actinomycetes Streptomyces and Nocardia. Less important than actinomycetes, fungi such as Trichoderma and Verticillium and bacteria, such as Bacillus and Pseudomonas can also degrade chitin.(textbook)
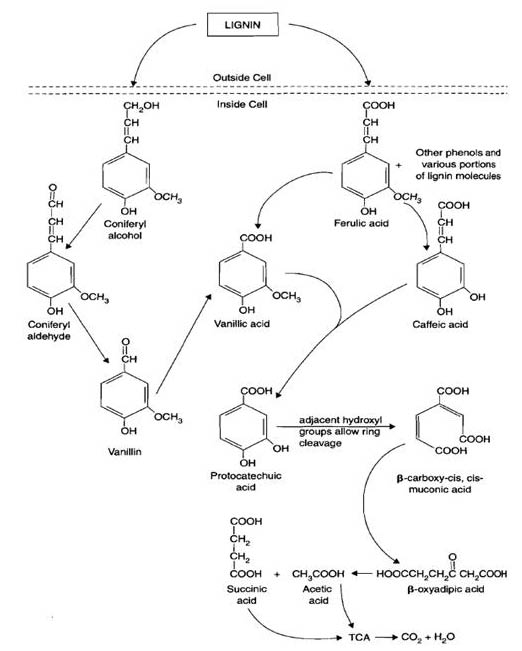
Lignin
Lignin is the main component of wood in trees. Lignin has a varied, unique, and complicated chemical structure which contains many aromatics. Aromatics are part of the reason why lignin is more difficult to be decomposed. Even with strong acid treatment, the plant residues are not solubilized due to the complex ring structure.These aromatics can be released from the lignin structure by fungal enzymes such as peroxidases and oxidases. The enzymes utilize H2O2 and OH radicals to break the bonds in the lignin. Lignin is decomposed through groups of specialized fungi. Common types of fungi which depolymerize lignin are white rot (Phanerochaete chrysosporium), brown rot, and soft rot. Once the aromatics are released from the original lignin structure they are incorporated into the metabolic pathway as pyruvate, acetyl CoA, and into the TCA cycle. Lignin stabilization through mineral interactions has recently been hypothesized to be a methodological artifact (Hernes et al. 2013).
Fats & Waxes
More than 2% of soil organic matter is cultivated in the forms, fats and waxes. Unlike cellulose and sugar, fats and waxes are much harder for microorganisms to breakdown. They are decomposed very slowly into CO2, water and energy which can be used by microorganisms. (6) Very little investigation has been done so information regarding this is limited.
Soil Organic Matter
The carbon cycle in soil is a dynamic balance between photosynthesis, the respiration of decomposing organisms, and the stabilization of carbon. Soil stores at least three times as much carbon (in SOM) as is found in either the atmosphere or in living plants (R). The total global carbon stock in soil is about 2500 Pg C, including approximately 1550 Pg in organic carbon, and 950 in inorganic carbon, as shown in figure #. Soils act as a buffer against the increase of atmospheric CO2. There is growing interest that it is possible to remove a significant amount of CO2 from the atmosphere by sequestering carbon in soil. It is estimated that 16-30% atmospheric CO2 can be removed when SOM concentrations increase 5-15% in soil up to 2m depth (Baldock, 2007; Kell 2011). It is also believed that soil respiration rates could cause a positive feedback in global warming. There are more nutrient based, plant-oriented interests in SOM, however, it seems that concerns about the changing climate “have now overtaken these other justifications”(Sollins 2007).
Growing Body of New Insights on SOM
For a long time, the scientific community described SOM in terms of humic substances. With the help of advanced analytical techniques, a growing body of research indicates that the importance of the biotic and abiotic environments in soil outweighs that of the molecular structure of plant inputs and organic matter in the process of OM stabilization. Factors such as rate of decay, SOM pools, stability, or ‘recalcitrance’ are not sufficient to describe the “ecosystem property” (Schmidt, 2011) of SOM; rather, texture, mineralogy, water solubility, molecular size, functionalization, and perhaps most important, climate change conditions should be incorporated into our understanding (and modeling) of carbon.
History of humic substances
Historically soil chemists use alkali and acid extraction methods and observations of the extracted (or residual) functional group chemistry to describe the soil organic matter, or humus. (Schmidt, et al, 2011) When protons are added to the solubilized organic materials, the dark color solid that precipitates is called ‘humic acid’. ‘Fulvic acid’ is the organic matter that remains soluble after reacidification treatment. The organic matter that does not respond to the extraction treatment is called ‘humin’. Long-standing theory suggests that humic substances are comprised of large and complex macromolecules which are the most stable SOM. Recently, by direct high-resolution observations, people understand that only a small portion of total organic matter is represented by humic substances. Smaller and simpler molecular structures are observed, as shown in Fig.1. The formation of humic polymers is not necessary related with humus formation in soils. A comprehensive review of traditional approach and critique of ‘humification’ model is conducted in the paper (Lehmann and Kleber, 2015). Biopolymer degradation and abiotic condensation (Frimmel & Christman, 1988) are the two general humification schemes developed on the knowledge of humus.
Decomposition and molecular structure
Historically SOM is thought to be made of stable and chemically unique compounds. The decomposition rate of plant residues largely depends on its biochemical composition, especially the C/N ratio and lignin content. Accordingly, people believe that the molecular structure of biomass and organic material controls the long-term decomposition rates in soil. Recent studies find that this is not true. Lignin and plant lipids (‘recalcitrants’) can turn over very rapidly. Moreover, sugar and other potential labile compounds have been shown to persist for decades---much longer than a couple weeks.
Atmospheric Changes Relating to the Carbon Cycle
Global Warming
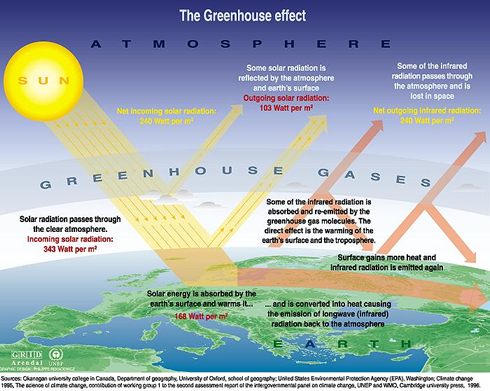
Global warming is a result of a greenhouse effect caused by the accumulation of greenhouse gases in the atmosphere. The earth is heated by solar radiation that penetrates the atmosphere and is absorbed by the earth. Some of the enegy is reflected back into the atmosphere. Greenhouse gases prevent the escape of this solar energy, causing increased heating of the earth, a phenomenon known as global warming. The major greenhouse gases are water vapour, nitrous oxide (N2O), carbon dioxide (CO2), methane (CH4), and ozone (O3). Soil microorganisms play a role in the generation of each of these gases [3].
Water Vapour
Water vapour accounts 36% to 66% of the greenhouse effect. The water vapor content of the atmosphere is expected to increase in response to an increase in temperatures. "The increased water vapor in turn leads to an increase in the greenhouse effect and thus a further increase in temperature; the increase in temperature leads to still further increase in atmospheric water vapor; and the feedback cycle continues until equilibrium is reached. Thus water vapor acts as a positive feedback to the driving force provided by human-released greenhouse gases such as CO2" [5][8].
Nitrous Oxide
Microorganisms produce N2O by performing aerobic nitrification and anaerobic denitrification. Nitrification is the conversion of ammonia to nitrate. Nitrobacter hamburgensis and Nitrosococcus oceani are examples of nitrifying bacteria. Denitrification is the reduction of nitrate to nitrogen gas, with N2O as a side product. Pseudomonas and Flavobacterium are examples of denitrifying bacteria [2]. For more information on nitrogen transformation, see the Nitrogen cycle including GHG.
Carbon Dioxide
Microorganisms produce CO2 through the decomposition of organic matter. The balance between microbial repiration of CO2 and plant fixation of CO2 is important when considering microbial contribution to the greenhouse effect [2].
Methane
Methane is the end product of anaerobic respiration. This process is called methanogenesis. Microorganisms that produce methane through respiration are called Methanogens. There are two processes by which methanogens produce methane: CO2 reduction and acetate fermentation [2].
Methanotrophy is the process by which microorganisms, such as Methylobacterium, utilize methane as a carbon source. Carbon dioxide is a product of the reaction. These microorganisms are called methanotrophs [2].
Acid Rain
Nitrous oxides not only contribute to the greenhouse effect, but also to acid rain. Acid rain is corrosive, and increases the acidity of surface water and soils. Basic soils, including soils containing substantial amounts of calcium carbonate, can neutralize the acid rain. These soils are common in the Midwest, Great Plains, and many Western states [1].
Surface water becomes acidified when the buffering capacity of the water and soil is overwhelmed by the acid rain. The acid rain liberates aluminum from the soil. The aluminum then enters the water, posing a serious threat to aquatic organisms. Acidification of soils, and the associated release of aluminum, also poses a threat to soil microorganisms. Low pH can also release toxic metals such as lead, mercury, zinc, copper, cadmium, chromium, manganese, and vanadium from the soil. These mobilized ions inhibit the growth of soil bacteria and fungi [1].
The effects of acid rain also accelerate calcium carbonate (CaCO3) degradation. CaCO3 reacts with the acid to form CaO and CO2. The CO2 is released from the soil and combines with water vapor in the atmosphere to form more acid rain. This process, which continues until CaCO3 is completely consumed, is an example of a positive environmental feedback loop.
Current Research
Professor Joshua Schimel is studying the effect of global warming on the soil organic matter in the Arctic. The increasing temperature could result in increased evolution of CO2 to the atmosphere. On the other hand, plant growth could be fostered by the increased presence of nutrients from soil organic matter, making the Arctic a sink for CO2. The nature of the organic matter present in the Arctic soils will determine the type of environmental feedback. http://www.lifesci.ucsb.edu/eemb/faculty/schimel/research/research.html
The Urban Water Research Center at UC Irvine is conducting research on the effect of stengthened anaerobic degradation of manure on methane generation. http://www.uwrc.uci.edu/research-urban-water/UCI-UWRC-Research-Areas.php
Recently the EPA released a report analyzing the existing data on the connections between UV radiation, global climate change, and biogeochemical cycling. The report found that increased UV radiation affected the cycling of nutrients such as carbon and nitrogen. UV radiation impacts root exudation and the properties of dead plant material that become incorporated into the soil. This impacts microorganisms, and in turn, biogeochemical cycling. http://www.ncbi.nlm.nih.gov/pubmed/17344963?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DiscoveryPanel.Pubmed_Discovery_RA
References
1."Acid Rain - Soil Interactions" Available from: http://www.elmhurst.edu/~chm/vchembook/196soil.html
2.Sylvia, D.M., Fuhrmann, J.J., Hartel, P.G.,& Zuberer, D.A. (2005).Principles and Applications of Soil Microbiology, Second Edition. Prentice Hall, Upper Saddle River, NJ, 346,359,497-501 and 285-287 pp.
3.http://www.blackwell-synergy.com/doi/abs/10.1046/j.1365-2486.1997.00100.x KEITH SMITH (1997) "The potential for feedback effects induced by global warming on emissions of nitrous oxide by soils" Global Change Biology 3 (4) , 327–338 doi:10.1046/j.1365-2486.1997.00100.x
4.http://www.lifestyleblock.co.nz/articles/organics/02_organic_soil_matter.htm
5.realclimate.org http://www.realclimate.org/index.php?p=142 "Water vapour: feedback or forcing?"
6.http://www.gfdl.noaa.gov/reference/bibliography/2006/ih0601.pdf Issac M. Held, Brian J. Soden. (2006) "Robust Responses of the Hydrological Cycle to Global Warming " Journal of Climate volume19,issue = 21,pages5686-5699
7.Erickson DJ 3rd, Paul ND, Sulzberger B, Zepp RG. "Interactive effects of solar UV radiation and climate change on biogeochemical cycling," Photochem Photobiol Sci. 2007 Mar;6(3):286-300. Epub 2007 Feb 6.PMID: 17344963 [PubMed - indexed for MEDLINE] http://www.ncbi.nlm.nih.gov/pubmed/17344963?ordinalpos=1&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DiscoveryPanel.Pubmed_Discovery_RA
8. http://en.wikipedia.org/wiki/Greenhouse_gas
Edited by student of Kate Scow